Bovine akabane disease virus vaccine
A technology for Akabane disease virus and vaccines, applied in the direction of viruses, virus peptides, antiviral agents, etc., can solve the problems of potential risks in vaccine safety, complex production processes, and many manpower and material resources, and achieve low production costs, high purity, The effect of strong antigen immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Vector construction and immunogenicity verification of G1 protein and G2 protein
[0026] 1. Construction of cell lines expressing G1 protein and G2 protein
[0027] 1.1 Gene synthesis
[0028] 1.1.1 G1 gene synthesis
[0029] The bovine Akabane disease gene sequence selected from Geenbank was compared and analyzed. According to the partial tropism of CHO cell codons, the codon optimization and modification of the G1 gene sequence was carried out, and the restriction site was designed, and the C-terminal of the G1 sequence was added. Different signal peptide sequences, and with a His tag, finally got SEQ ID NO.1.
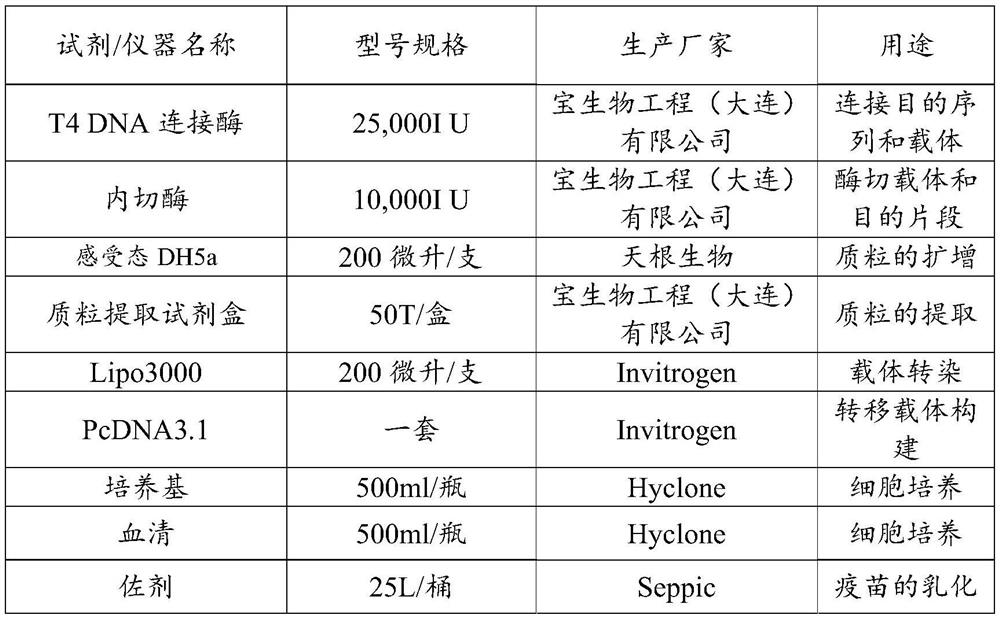
[0030] The nucleotide sequence encoding the G1 protein was inserted into the eukaryotic transfer vector pcDNA3.1 through the BamHI and NotI sites, ligated with T4 DNA ligase overnight at 16°C, transformed with Escherichia coli competent DH5a and coated on On the LB plate containing ampicillin, pick positive colonies after culturing overnight at 3...
Embodiment 2
[0046] Determination of Example 2 Antigen Ratio
[0047] After decellularization, the expressed proteins were quantified separately, and mixed with appropriate adjuvants to prepare vaccines. The final finished vaccines contained different doses of G1+G2 proteins (10μg+10μg, 20μg+20μg, 30μg+30μg, 40μg+40μg, 50μg+50μg, 60μg+60μg, 70μg+70μg, 80μg+80μg) / mL, immunize 3-4 month old calves (healthy susceptible, AKAV antibody negative), 2ml / head, 21 days after immunization, blood test for neutralizing antibody To determine the optimal immunization dose, the results showed that the fifth group (50μg+50μg) could produce good neutralizing antibodies, the sixth group, the seventh group, and the eighth group (60μg+60μg, 70μg+70μg, 80μg+80μg) And the antibody highest value, lowest value, average value to analyze, three neutralizing antibody titer differences are not significant, in order to ensure the effectiveness of this invention product, the antigen dose in this invention is determined ...
Embodiment 3
[0050] The immunoprotective effect of embodiment 3 vaccine
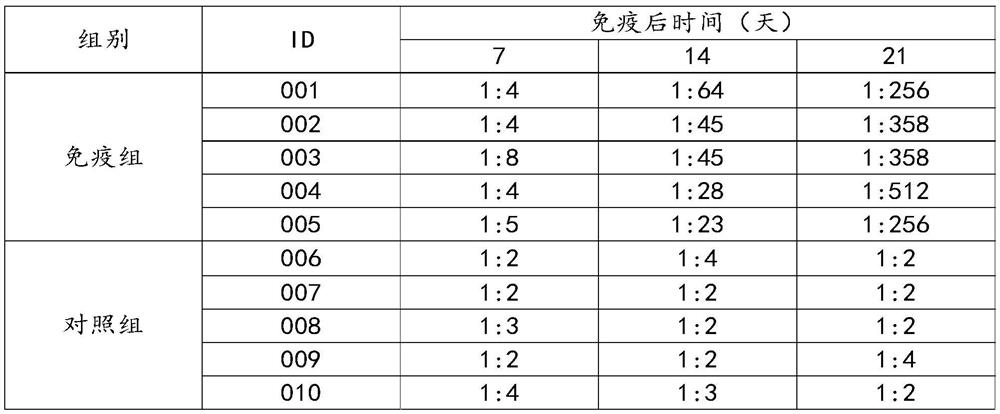
[0051] After decellularized fragments, the expressed proteins were quantified separately, and then mixed with appropriate adjuvants to prepare vaccines. The final protein content of G1 and G2 in the finished vaccine was 60 μg+60 μg / mL. Ten healthy 3-4 month-old calves (healthy and susceptible, AKAV antibody negative) were used, among which 5 calves were immunized with 2ml / head of the vaccine, and the other 5 calves were immunized with the same dose of normal saline as a control group. On the 7th, 14th, and 21st day after immunization, and on the 7th and 14th day after the challenge, the blood of the immunized group and the control group was collected, and the neutralizing antibody of bovine Akabane disease virus was measured respectively. The test results are shown in Tables 4 and 5.
[0052] After the immunization, the virus was challenged at the same time, and the oral and anal swabs were collected after the challe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com