Combined vaccine bonding bacterial polysaccharides and protein for human
A combined vaccine and bacterial polysaccharide technology, applied in the direction of antibacterial drugs, bacterial antigen components, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of Capsular Polysaccharides of Group A and C Meningococci and Capsular Polysaccharide of Haemophilus Influenzae Type B Conjugate Vaccine
[0021] Prepare group A meningococcal capsular polysaccharide-TT conjugate vaccine stock solution, group C meningococcal capsular polysaccharide-TT conjugate vaccine stock solution, b Haemophilus influenzae type capsular polysaccharide-TT conjugate vaccine stock solution. Dilute group A meningococcal capsular polysaccharide-TT conjugate vaccine stock solution, group C meningococcal capsular polysaccharide-TT conjugate vaccine stock solution, and Haemophilus influenzae type b capsular polysaccharide-TT conjugate vaccine stock solution to 60-120 μg respectively / ml (according to polysaccharide calculation), add the above three diluted vaccine solutions into a sterile container according to the required amount, after mixing thoroughly, add a certain amount of sterile pyrogen-free sodium chloride and aluminum hydroxide Adjuva...
Embodiment 2
[0023] HibACon TM Clinical Research Trials in Children 1-2 Years
[0024] 3 batches of Group AC meningococcus-Haemophilus influenzae type b capsular polysaccharide conjugate vaccine prepared in the GMP workshop according to the above-mentioned Example 1 method according to GMP requirements, each milliliter of the vaccine contains Group A and Group C meningococcal capsular polysaccharides 20 μg each of Haemophilus influenzae type b capsular polysaccharide. The State Food and Drug Administration has randomly inspected a batch of vaccines for clinical trials.
[0025]The clinical research was carried out by double-blind method, and the vaccinated people who met the age group of enrollment were all never vaccinated with group A or group A+C meningococcal polysaccharide vaccine and Haemophilus influenzae type b conjugate vaccine.
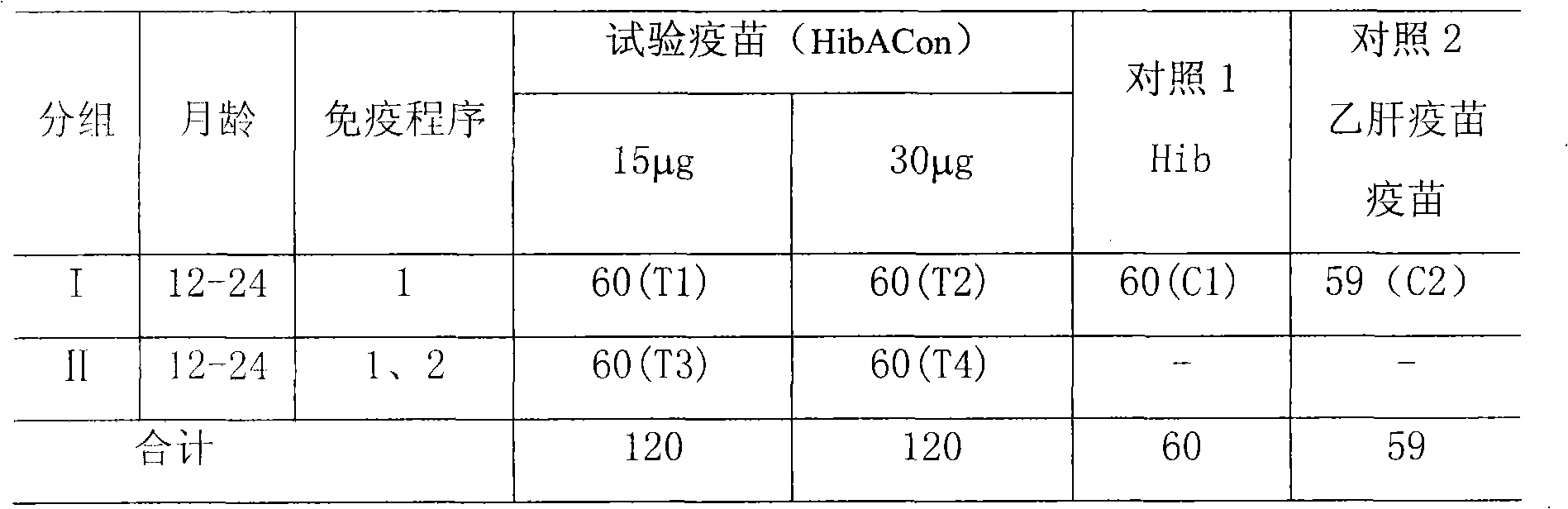
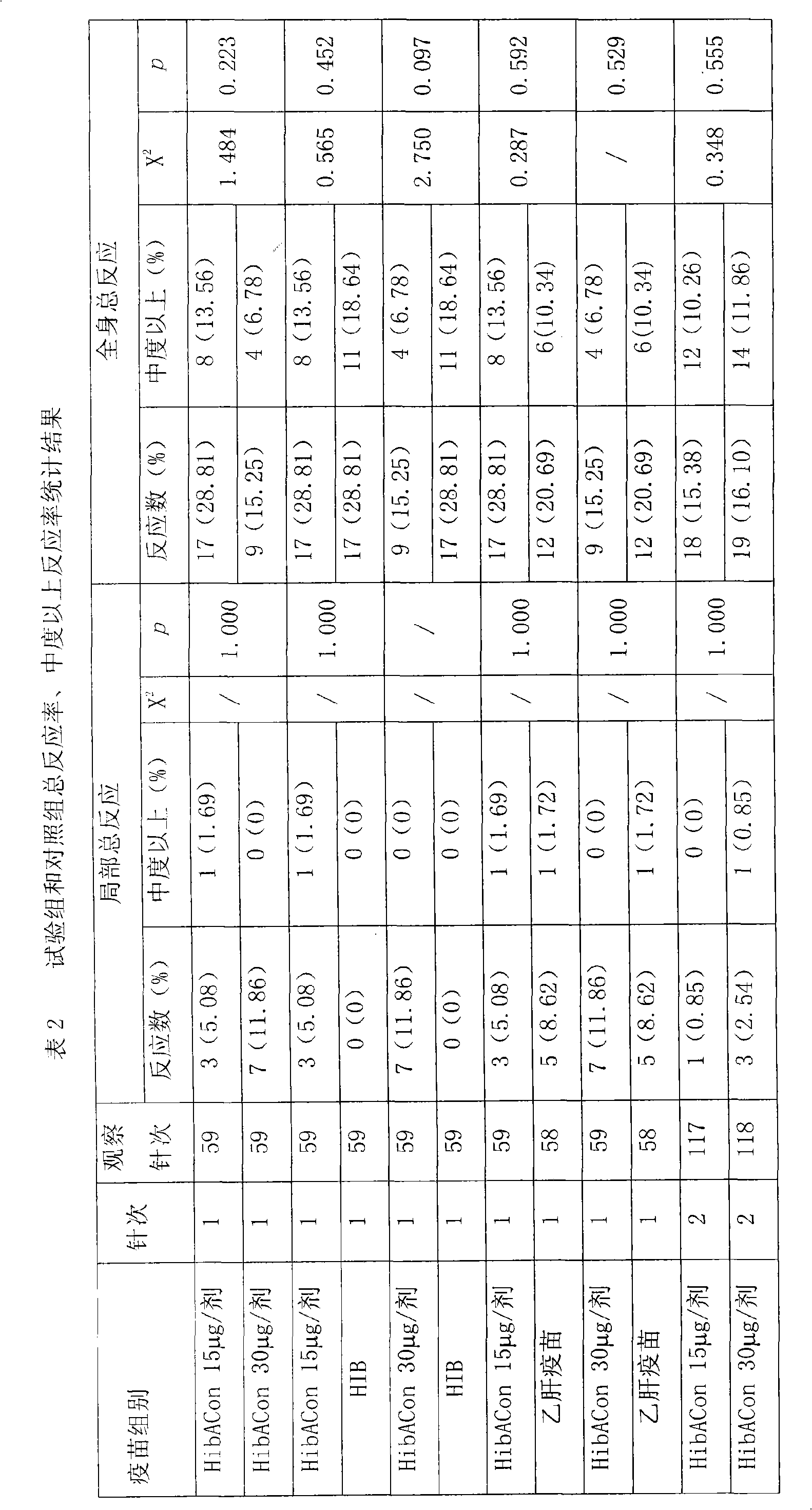
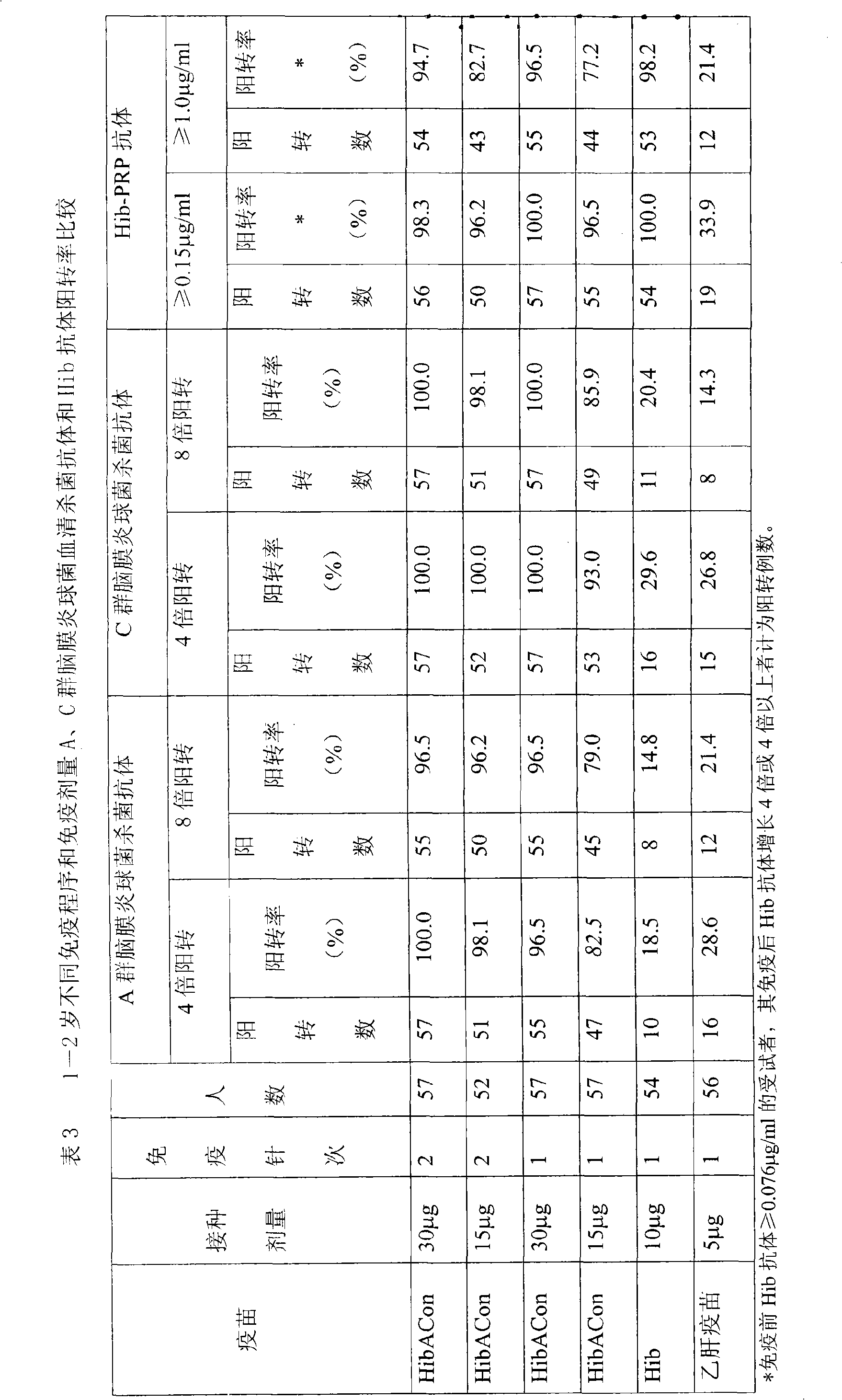
[0026] The clinical study was divided into 4 research groups (two different immunization program groups, each program group was inoculated with differ...
Embodiment 3
[0038] HibACon TM Clinical research trials in infants 3-8 months of age
[0039] 3 batches of AC group meningococcus-type b Haemophilus influenzae capsular polysaccharide conjugate vaccine prepared according to the method of above-mentioned embodiment 1, each dose of HibACon vaccine contains A group, C group meningococcal capsular polysaccharide, b type haemophilus Each 10 μg of Bacillus influenzae capsular polysaccharide.
[0040] The clinical research is carried out by double-blind method. All the vaccinated people in the age group who met the enrollment had never been vaccinated with group A or group A+C meningococcal polysaccharide vaccine and Haemophilus influenzae type b conjugate vaccine. The clinical study was divided into two research groups, each group was inoculated with different doses (15 μg, 30 μg) of the research vaccine, and the control group was the Haemophilus influenzae type b conjugate vaccine. Infants aged 3-8 months were inoculated 3 injections, with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com