Combined hepatitis A and B vaccine and preparation method thereof
A hepatitis B and combined vaccine technology, applied in biochemical equipment and methods, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as complex immunization procedures and increased immunization costs, and achieve good safety and improve immunity. The success rate of inoculation and the effect of reducing the number of inoculations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
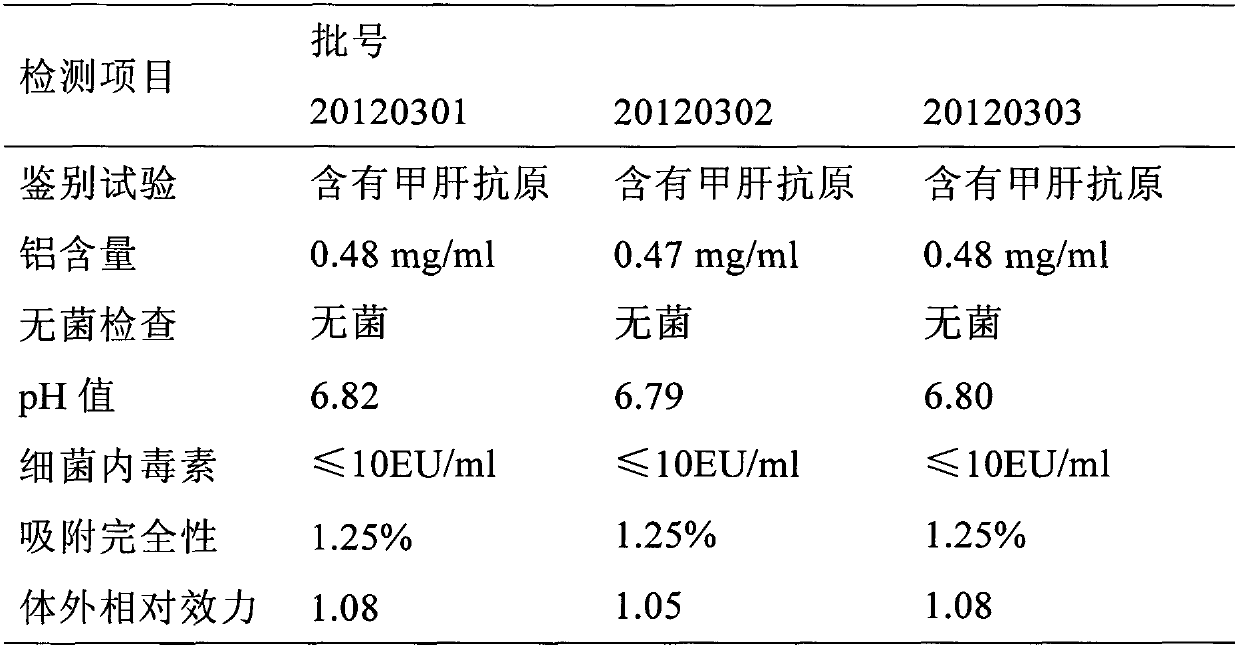
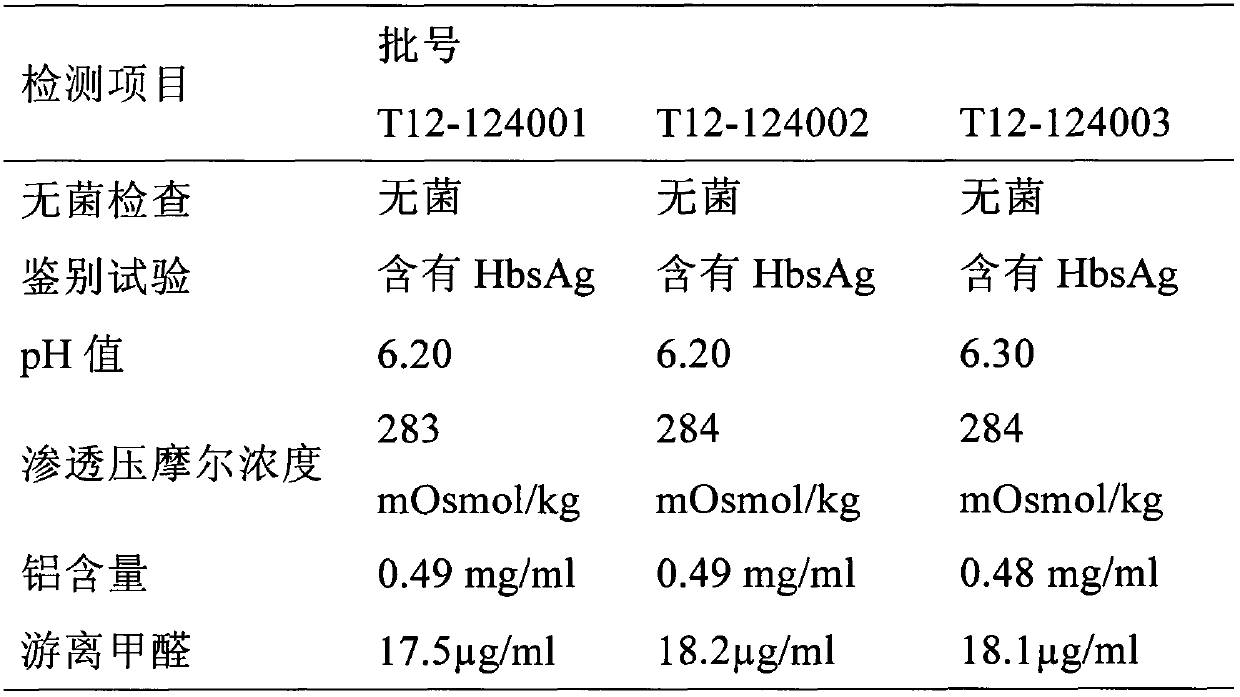
[0030] This embodiment mainly includes hepatitis A virus antigen and hepatitis B virus surface antigen protein, wherein, the hepatitis A virus antigen is the hepatitis A virus harvested and purified by human embryo lung diploid cell culture Viral antigen, the content of which is 630-660EU / ml. The content of the hepatitis B virus surface antigen protein is the hepatitis B virus surface antigen protein expressed and purified by recombinant Saccharomyces cerevisiae, and the content is 15-30 μg / ml.
[0031] In addition, this embodiment may also include immune aluminum adjuvant, and the content of immune aluminum adjuvant is 0.35-0.62 mg / ml.
Embodiment 2
[0033] This embodiment mainly includes hepatitis A virus antigen and hepatitis B virus surface antigen protein, wherein, the hepatitis A virus antigen is the hepatitis A virus harvested and purified by human embryo lung diploid cell culture Viral antigen, its content is 640EU / ml, the content of the hepatitis B virus surface antigen protein is the recombinant Saccharomyces cerevisiae fermented and expressed and purified hepatitis B virus surface antigen protein, its content is 20μg / ml, the content of immune aluminum adjuvant 0.35 ~ 0.62mg / ml.
[0034] During specific implementation, the hepatitis A virus strain SH strain used in this embodiment is a strain newly isolated by our company with high toxin production, short culture period, and good adaptation to human embryonic lung diploid cells, which can effectively increase the Yield and safety are better; cell-virus mixed adsorption technology is used to greatly increase virus yield, shorten virus culture cycle, and cell factor...
Embodiment 3
[0038] This embodiment mainly includes the following steps:
[0039] The preparation step of the hepatitis A virus antigen is to harvest and purify the hepatitis A virus through human embryonic lung diploid cells to obtain the hepatitis A virus antigen;
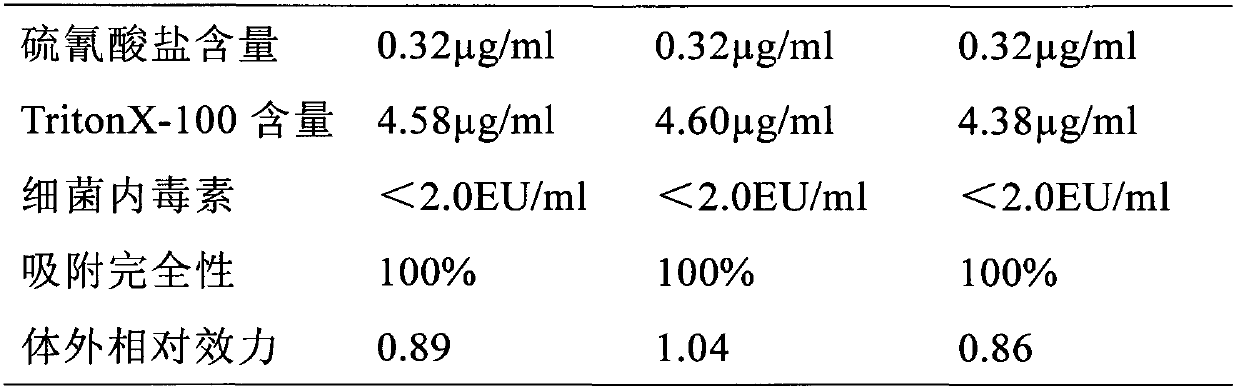
[0040] The preparation step of hepatitis B virus surface antigen protein, fermenting and purifying recombinant Saccharomyces cerevisiae, hepatitis B virus surface antigen protein;
[0041] In the mixing step, the hepatitis A virus antigen and the hepatitis B virus surface antigen protein are mixed, and the pH value is adjusted to between 6.0 and 7.0 to prepare a combined hepatitis A and hepatitis B vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com