Vaccine composition, preparation method and application thereof
A vaccine composition and composition technology, applied in the field of vaccine composition, can solve problems such as inability to vaccine immune resistance, drug-resistant drugs, and decline in the quality of immune prevention effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 mycoplasma hyopneumoniae antigen, swine influenza virus antigen and porcine blue ear virus antigen
[0052] 1. Preparation of Mycoplasma hyopneumoniae antigen
[0053] 1.1 Source of the strain:
[0054] The strain of Mycoplasma hyopneumoniae used in the manufacture and inspection of this product is HN0613, which was preserved in the China Center for Type Culture Collection (abbreviation: CCTCC; address: Wuhan University, Wuhan, China). The preservation date: June 13, 2012, and the preservation number: CCTCC NO: M2012230.
[0055] 1.2 Preparation of seeds for Mycoplasma hyopneumoniae production:
[0056] Propagation of primary seeds: freeze-dried strain (HN0613 strain, preservation number CCTCC No.M2012230), diluted with liquid medium, streaked and inoculated on a solid medium plate, cultured at 37°C for 7 days, and selected well-growing colonies , inoculated on the slant of solid medium, cultivated at 37°C for 7 days, and used as first...
Embodiment 2
[0076] The preparation of embodiment 2 mycoplasma pneumonia, swine influenza and porcine PRRS triple vaccine
[0077] 1. Preparation of parts of mycoplasma swine pneumonia and swine influenza inactivated vaccines
[0078] 1.1 Preparation of diluent
[0079] Sterile PBS buffer solution: Dissolve 8g sodium chloride, 0.25g potassium chloride, 3.63g disodium hydrogen phosphate, 0.24g potassium dihydrogen phosphate in 900ml purified water, then dilute to 1L, autoclave at 121°C for 30min spare.
[0080] 1.2 Vaccine adjuvant treatment
[0081] Sterilization of Gel 01 adjuvant: transfer the Gel 01 adjuvant into a sterilizable container, and autoclave at 121°C for 30 minutes for later use.
[0082] 1.3 Matching seedlings
[0083] Through aseptic operation, the concentrated Mycoplasma hyopneumoniae antigen and swine influenza virus antigen prepared in Example 1 were mixed with adjuvants, preservatives, diluents, etc. according to different proportions, and stirred at a low speed by ...
Embodiment 3
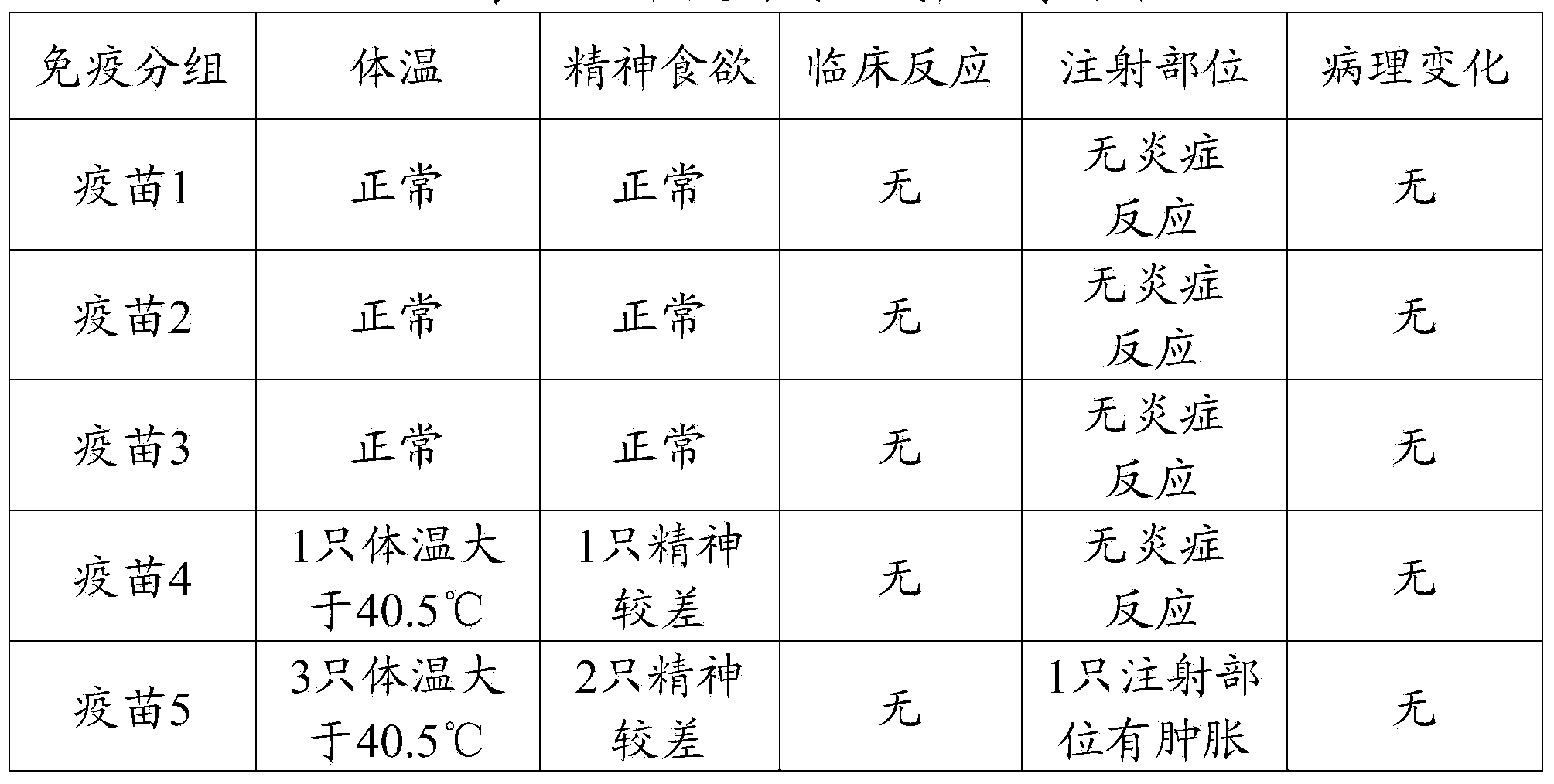
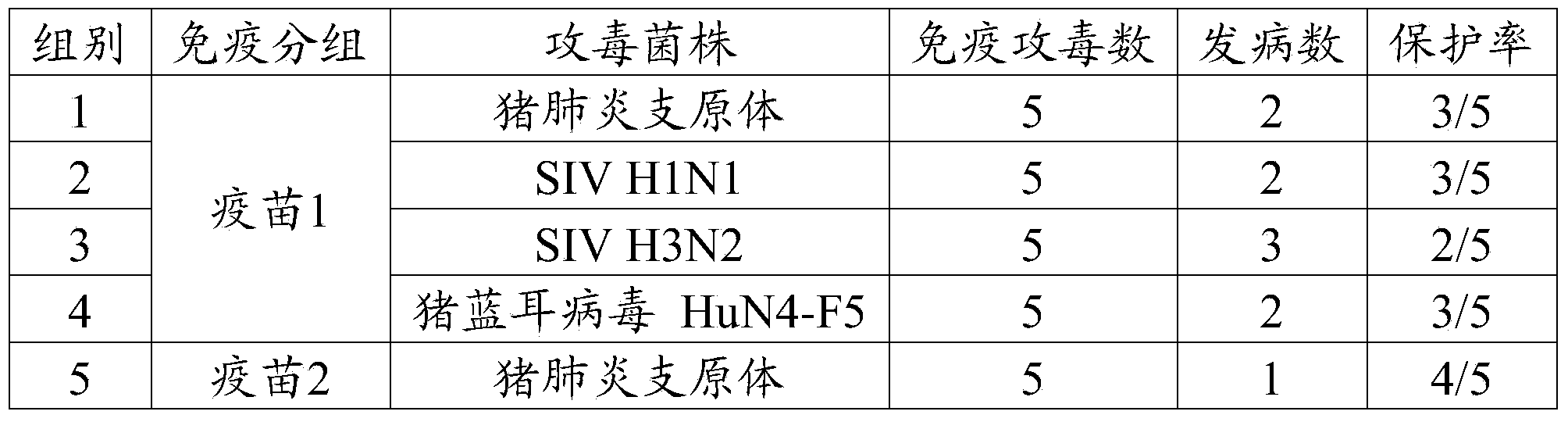
[0090] Example 3 Efficacy Test of Triple Vaccine of Mycoplasma Swine Pneumonia, Swine Influenza and Pig PRRS with Different Antigen Contents
[0091] 1 test material
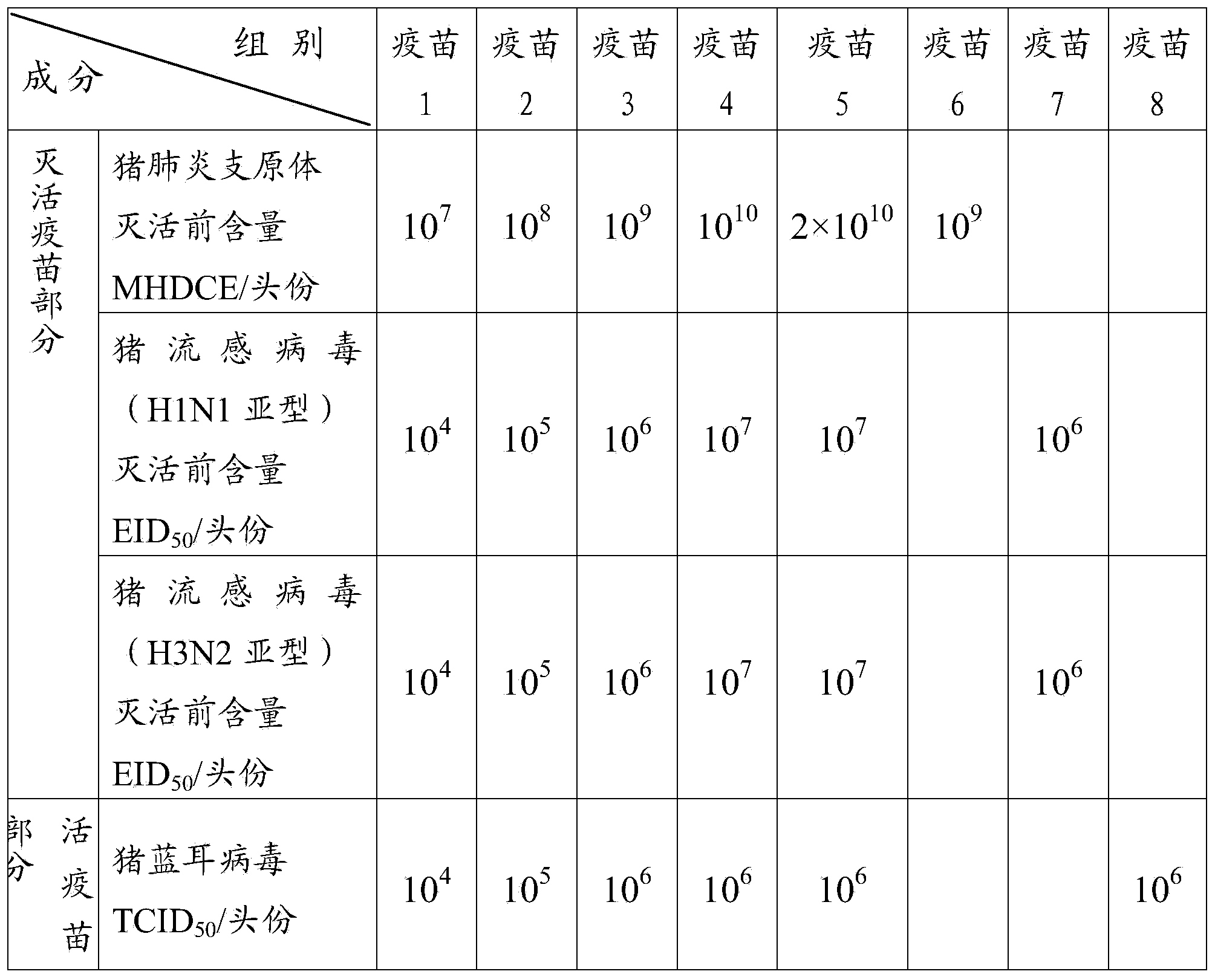
[0092] Vaccine 1 (mycoplasma hyopneumoniae 10) prepared in embodiment 2 7 MHDCE / toufen, swine influenza virus (H1N1 subtype) and swine influenza virus (H3N2 subtype) each 10 4 EID 50 / Toufen, porcine blue ear virus 10 4 TCID 50 / head), vaccine 2 (Mycoplasma hyopneumoniae 10 8 MHDCE / toufen, swine influenza virus (H1N1 subtype) and swine influenza virus (H3N2 subtype) each 10 5 EID 50 / Toufen, porcine blue ear virus 10 5 TCID 50 / head), vaccine 3 (Mycoplasma hyopneumoniae 10 9 MHDCE / toufen, swine influenza virus (H1N1 subtype) and swine influenza virus (H3N2 subtype) each 10 6 EID 50 / Toufen, porcine blue ear virus 10 6 TCID 50 / head), vaccine 4 (Mycoplasma hyopneumoniae 10 10 MHDCE / toufen, swine influenza virus (H1N1 subtype) and swine influenza virus (H3N2 subtype) each 10 7 EID 50 / Toufen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com