Combined vaccine, and preparation method and application thereof
A combination vaccine and inactivation technology, applied in the biological field, can solve the problems of inability to use attenuated vaccines in combination, differences in mechanism of action, cumbersome vaccination procedures, etc., and achieve the effect of reducing the number of vaccinations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention also includes a preparation method of the combined vaccine, the preparation method comprising the following steps:
[0061] Preparation of inactivated varicella-zoster virus purified liquid;
[0062] Prepare the purified solution of inactivated Japanese encephalitis virus;
[0063] Mix the inactivated Japanese encephalitis virus purified liquid and the inactivated varicella-zoster virus purified liquid,
[0064] Sterilize and freeze-dry the mixed purified virus liquid to obtain the combined vaccine.
[0065] The steps for preparing the purified solution of inactivated varicella-zoster virus are as follows:
[0066] Human diploid cells are subcultured;
[0067] Inoculating the varicella-zoster virus onto the subcultured human diploid cells, and culturing the virus to obtain a varicella-zoster virus harvest liquid;
[0068] Concentrate the varicella-zoster virus harvest liquid by ultrafiltration until the total protein content in the concentrated...
Embodiment 1
[0094] Embodiment 1 The preparation method of inactivated varicella-zoster virus purified liquid
[0095] Experimental materials and sources:
[0096] Human diploid ZFB-3 cells;
[0097] Varicella zoster virus, clinical collection;
[0098] 0.2%~0.5% human serum albumin, purchased from Tonglu Biopharmaceutical;
[0099] MEM medium, purchased from Thermo company;
[0100] PBS, homemade;
[0101] Ultrafiltration membrane bag, purchased from Millipore;
[0102] β-propiolactone, purchased from Serva company;
[0103] Preparation:
[0104] 1. Passage ZFB-3 cells to 20 generations in cell culture flasks and cell factories, wherein ZFB-3 cells are host cells;
[0105] 2. When the host cell is amplified to a fusion degree of more than 90%, the varicella-zoster virus is inoculated into the host cell for infection, and the M.O.I. is 0.001;
[0106] 3. Add MEM medium containing 0.2% to 0.5% human serum albumin, and place it in a cell incubator at 34°C for cultivation;
[0107] 4...
Embodiment 2
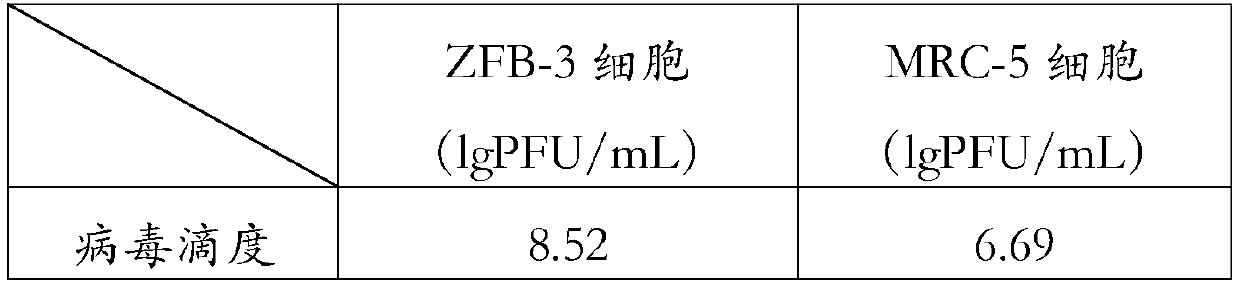
[0113] The adaptation of embodiment 2 Japanese encephalitis virus on ZFB-3 cell
[0114] Experimental materials and sources:
[0115] Human diploid ZFB-3 cells;
[0116] Japanese encephalitis virus (JEV) P3 strain was purchased from China National Institutes for Food and Drug Control
[0117] Fetal bovine serum was purchased from GIBCO and Hangzhou Sijiqing;
[0118] MEM medium, purchased from Thermo company;
[0119] PBS, Homemade.
[0120] experiment procedure:
[0121] The mouse brain-derived Japanese encephalitis virus P3 strain was adapted to MRC-5 cells and ZFB-3 cells respectively;
[0122] Using the method of multiple screening of virus plaques, the P3 virus strains should not be less than 10 -3 After doubling dilution, they were seeded in 96-well plates of monolayer cells, and MRC-5 cells and ZFB-3 cells were respectively set to correspond to 96-well plates. Adaptation on MRC-5 cells and ZFB-3 cells was carried out simultaneously under the same conditions.
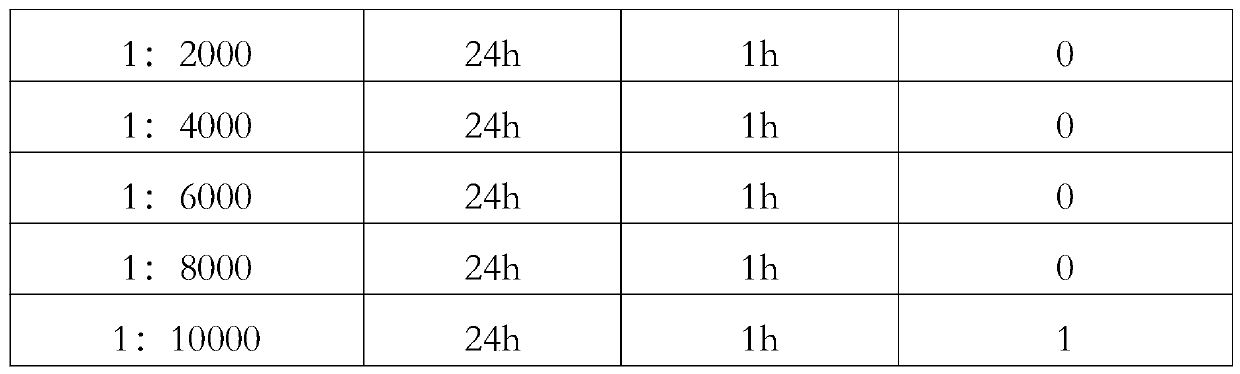
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com