Mink hemorrhagic pneumonia and botulism combined inactivate vaccine and preparing method thereof
A dual inactivated vaccine, hemorrhagic pneumonia technology, applied in vaccines, multivalent vaccines, veterinary vaccines, etc., can solve problems such as labor, increased cost, and impact on immune effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] - Bacteria inspection
[0067] 1. Morphological and biochemical properties
[0068] Pseudomonas aeruginosa strain G type WD005, type B DL007 strain, and type C strain ZC118 were Gram-negative, short bacilli with obtuse round ends. The biochemical characteristics conform to the characteristics of this bacteria in bacterial taxonomy.
[0069] Clostridium botulinum type C strain C62-4 is a Gram-positive large bacillus, and old cultures often become Gram-negative, producing atopic spores. The biochemical characteristics should conform to the characteristics of the bacteria in bacterial taxonomy.
[0070] 2. Cultivate traits
[0071] Pseudomonas aeruginosa G-type WD005, B-type DL007, and C-type ZC118 grow on nutrient agar plates as round, smooth, moist, flat colonies; when grown on MacConkey medium, the colonies are off-white; On the sheep blood agar plate, it can produce obvious β-hemolysis; when it grows on the medium for the determination of pyocyanin (PDP), it will p...
Embodiment 3
[0097] - vaccine preparation
[0098] 1. Preparation of Pseudomonas aeruginosa bacterial solution
[0099] (1) Primary seed propagation and identification Pseudomonas aeruginosa G type WD005 strain, B type DL007 strain, and C type ZC118 strain were cultured respectively. Inoculate strains into improved Martin broth medium, culture at 37°C for 12-18 hours, streak inoculate nutrient agar plate containing 5% newborn bovine serum, culture at 37°C for 18-36 hours, pick 5-10 typical colonies, inoculate Several branches of sheep blood agar slant were cultured at 37°C for 18-36 hours as primary seeds. Sampling shall be pure with a nutrient agar plate containing 5% newborn bovine serum for pure inspection. Store at 2-8°C, and the service life should not exceed 14 days.
[0100] (2) Secondary seed propagation and identification Pseudomonas aeruginosa G type WD005 strain, B type DL007 strain, and C type ZC118 strain were cultured respectively. The primary seeds were inoculated into t...
Embodiment 4
[0122] - Vaccine testing
[0123] 1. Properties After standing still, the upper layer is a clear liquid, and the lower layer is a gray-white precipitate, which becomes a uniform suspension after shaking.
[0124] 2. The inspection of the filling capacity shall be carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and all of them are in compliance with the regulations.
[0125] 3. The sterility test was tested according to the appendix of the current "Chinese Veterinary Drug Code", and all of them grew aseptically.
[0126] 4. Choose one of the following methods for safety inspection.
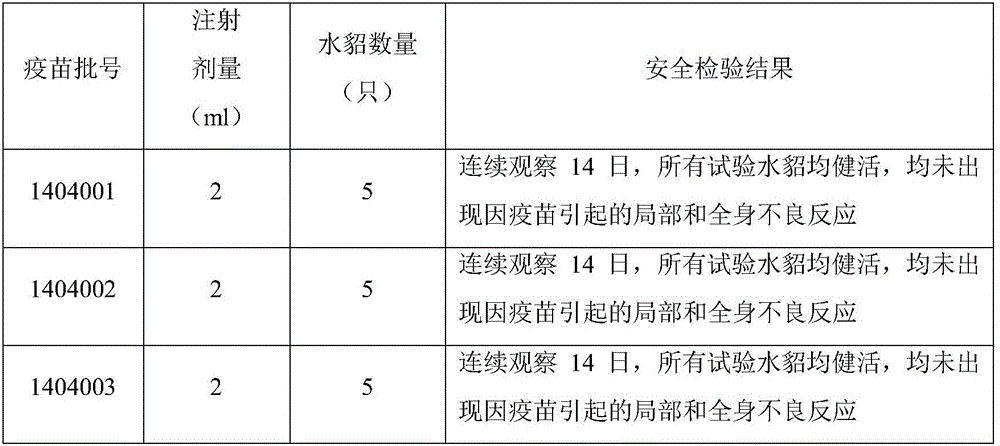
[0127] (1) Three batches of laboratory products were tested with mink and injected subcutaneously into 2-month-old healthy susceptible mink (Pseudomonas aeruginosa type G, B, and C slide agglutination test antibodies were negative; Clostridium botulinum neutralizing antibodies were negative ) of 5 minks, 2.0ml of the vaccine was subcutaneously injected int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com