A kind of freeze-dried preparation of Japanese encephalitis vaccine
A technology of freeze-dried preparations and vaccines, which is applied in the field of virus biology, can solve problems such as the impact on product safety, and achieve the effect of simple composition and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1.1 Prepare the vaccine semi-finished product according to the following formula, and test its freeze-drying effect:
[0026] Human serum albumin 0.5(w / w)%;
[0027] Mannitol 5.0(w / w)%;
[0028] JE virus antigen stock solution 50mL;
[0029] Supplement the pH7.2~8.0 phosphate buffer to 100mL.
[0030] 1.2 Preparation method of Japanese encephalitis vaccine
[0031] The purified Japanese encephalitis virus antigen stock solution is prepared into a semi-finished product according to the above formula;
[0032] The semi-finished product is divided into 2ml vials, 0.5ml per tube, and placed in a small lyophilizer for freeze-drying.
[0033] 1.3 Vaccine testing method
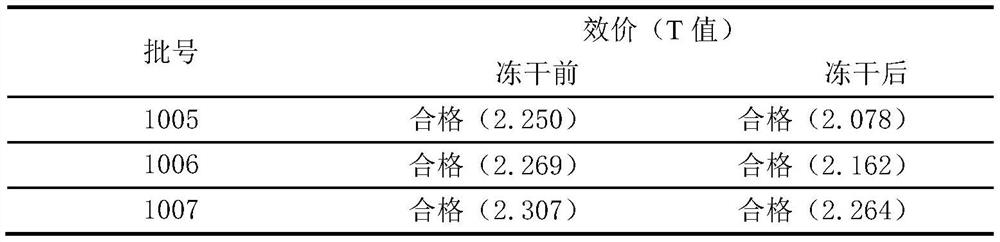
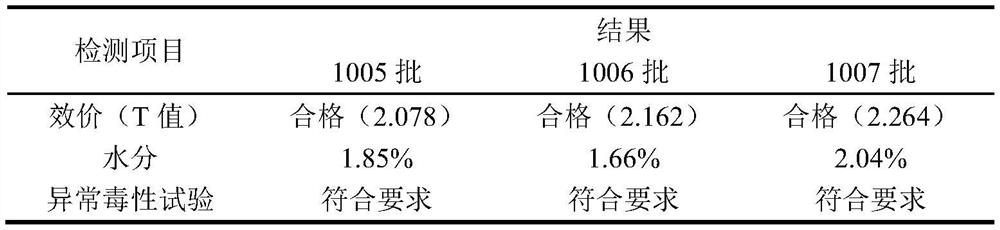
[0034] Potency determination: according to "Chinese Pharmacopoeia" 2010 edition three freeze-dried Japanese encephalitis inactivated vaccine (Vero cell) potency determination method;
[0035] Moisture: Measured according to the method VIID of the third appendix of "Chinese Pharmacopoeia" 2010 edition; ...
Embodiment 2
[0064] 2.1. Effect of different dosages of mannitol on vaccine preparations
[0065] Set the concentration of human serum albumin to 0.5%, and adjust the concentration of mannitol therein, and observe the influence of the concentration of mannitol on the vaccine. The specific comparison results are shown in Table 5:
[0066] The influence of table 5 different mannitol concentrations on freeze-drying effect
[0067]
[0068] The data in Table 5 shows that within the concentration range of 1.0-10% mannitol, the appearance, reconstitution time and water content all meet the relevant requirements of "Chinese Pharmacopoeia" three (2010 editions) to freeze-dried JE vaccine, but in 3 Both the appearance and the reconstitution time performed best in the concentration range of ~6% mannitol.
[0069] 2.2 Effects of different dosages of human serum albumin on vaccine preparations
[0070] Set the concentration of mannitol to 5%, adjust the concentration of human serum albumin therein...
Embodiment 3
[0074] The impact of the vaccine stabilizer of embodiment 3 different combinations on the vaccine preparation
[0075]
[0076]
[0077] The above experiments show that the vaccine stabilizer of the present invention effectively controls the loss rate of vaccine activity in the freeze-drying process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com