Vaccine protection agent, and combined measles and Japanese encephalitis vaccine and preparation method thereof
A vaccine protective agent and measles technology, which is applied in the field of protective agent of measles and Japanese encephalitis combined vaccine, can solve the problems of poor stability, short validity period and high adverse reaction rate, and achieve good stability, extended shelf life and good protection effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 protective agent of the present invention
[0027] 1. Preparation method
[0028] (1), gelatin solution: dissolve gelatin in sterilized phenol red-free EBSS balanced salt solution under the condition of 100 ℃ hot water bath to aid dissolution, stir in real time, wait until completely dissolved and clear, then make the gelatin concentration 5% ~25%, 0.11MPa for 30 minutes to sterilize, then dilute to volume with sterilized phenol red-free EBSS balanced salt solution in a sterile environment to obtain a gelatin solution, store at 37°C until use;
[0029] (2) Sugar solution: Dissolve trehalose and sucrose in sterilized EBSS balanced salt solution under the condition of 100°C hot water bath to aid dissolution, stir in real time, and after being completely dissolved and clear, sterilize at 100°C for 30 minutes, Constant volume, so that the concentration of each sugar in the solution is 20-40%, while hot, sterilize and filter to obtain the sug...
Embodiment 2
[0032] The screening experiment of embodiment 2 protective agent of the present invention
[0033] 1. Preparation method
[0034] (1), preparation of protective agent
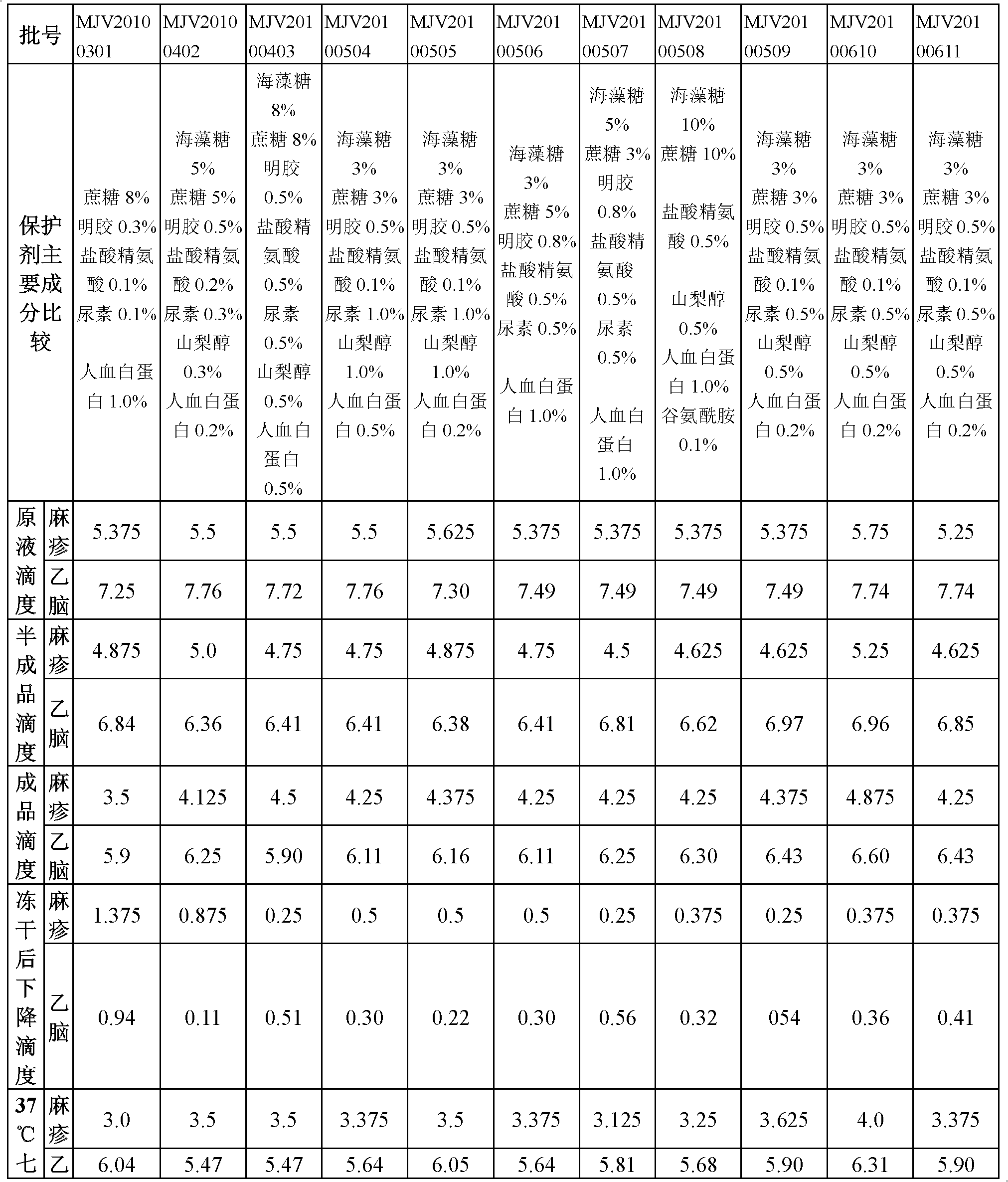
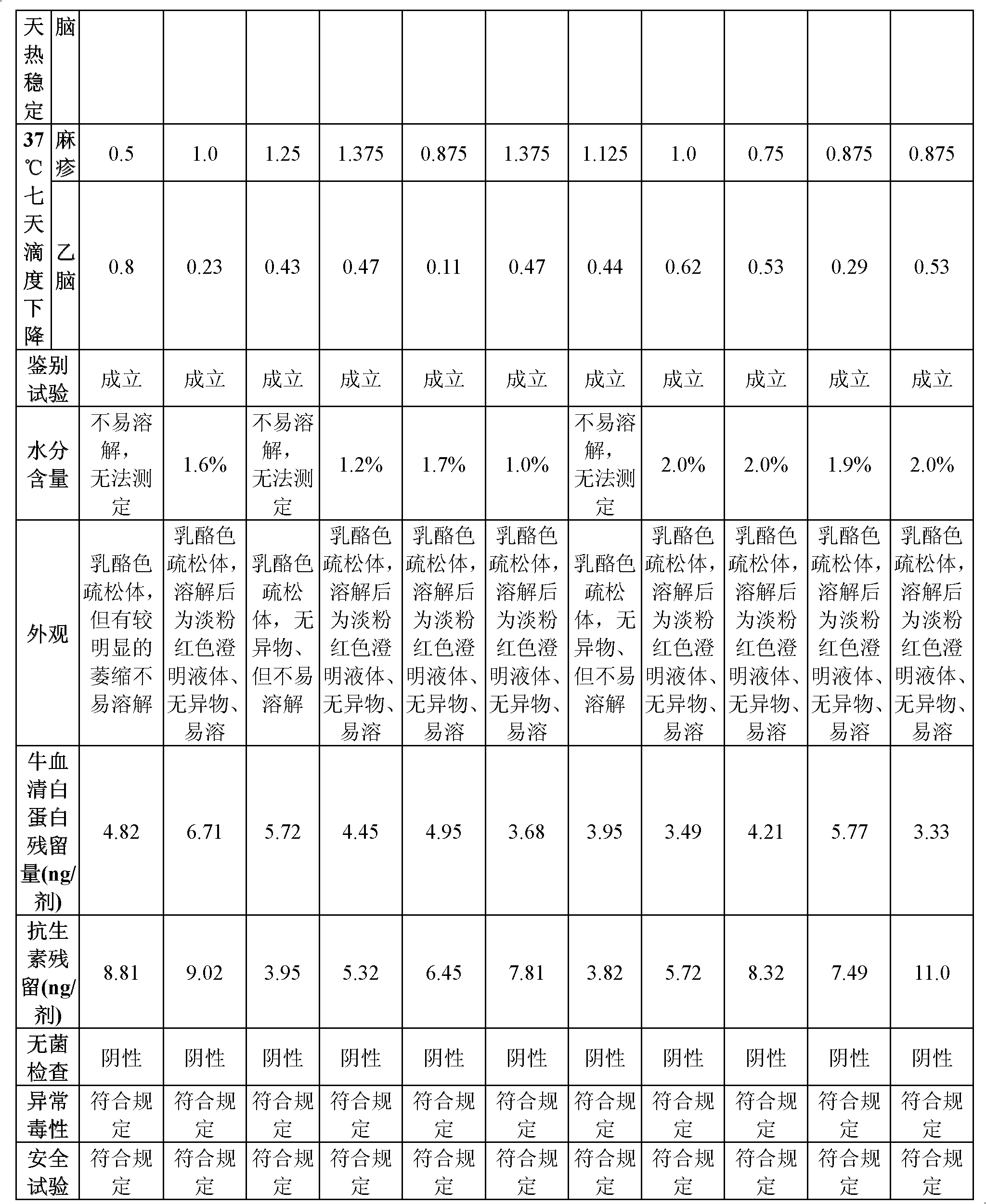
[0035] According to the formula shown in Table 1, the protective agent was prepared according to the following steps:
[0036] a. Gelatin solution: Dissolve gelatin in sterilized phenol red-free EBSS balanced salt solution under the condition of 100°C hot water bath to aid dissolution, stir in real time, wait until it is completely dissolved and clear, and then set the volume to make the gelatin concentration 5% to 25% %, 0.11MPa for 30 minutes to sterilize, then dilute to volume with sterilized phenol red-free EBSS balanced salt solution in a sterile environment to obtain a gelatin solution, and store it at 37°C for later use;
[0037] b. Sugar solution: Dissolve trehalose and sucrose in sterilized EBSS balanced salt solution under the condition of 100°C hot water bath to aid dissolution, stir in real time, ...
Embodiment 3
[0064] Embodiment 3 vaccine stability test of the present invention
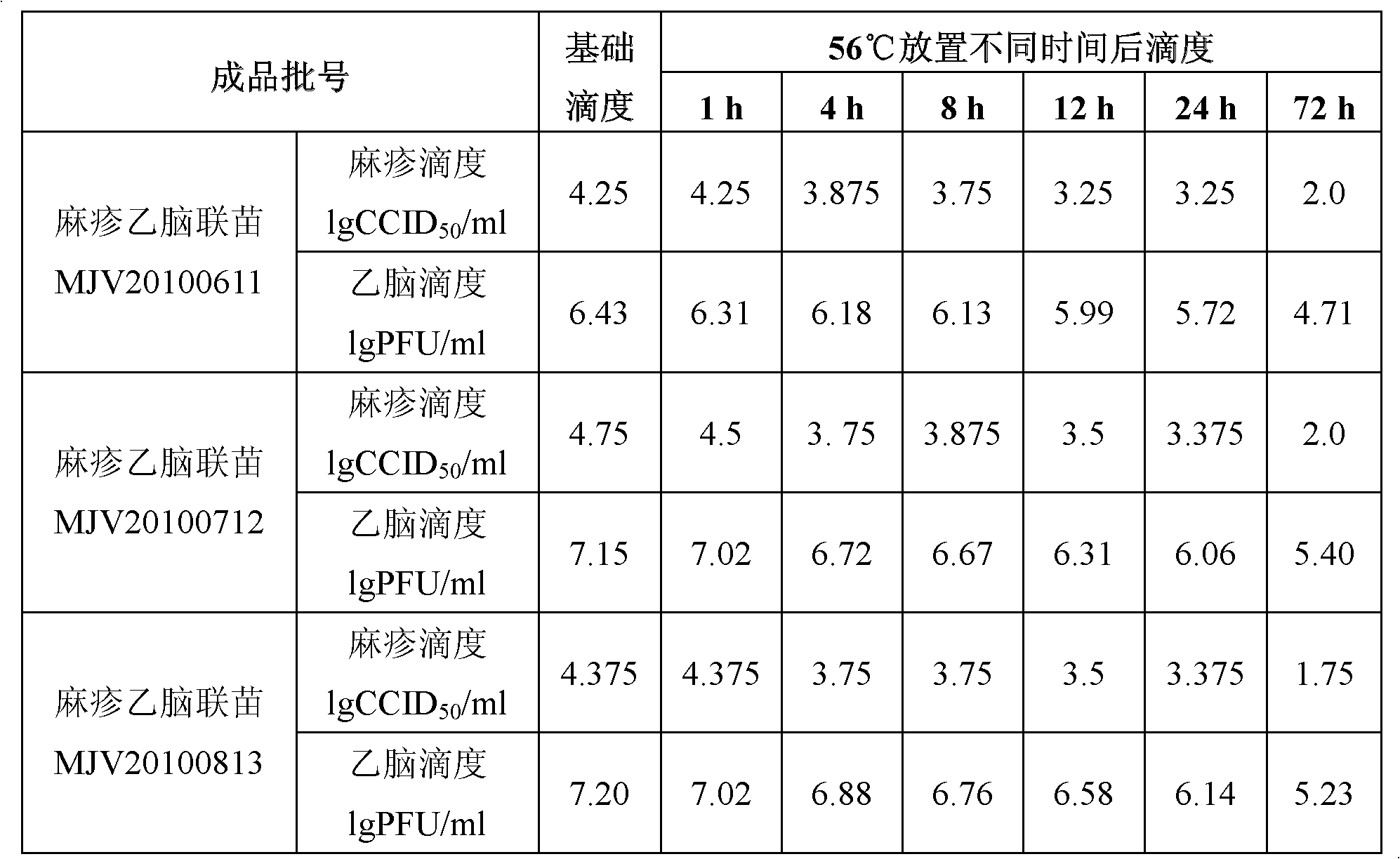
[0065] Take the batch number MJV20100611 prepared in Example 2 and the three batches of JE-Measles combined vaccines, MJV20100712 and MJV20100813, which are completely consistent with the formula of the protective agent in Example 2, and refer to ICH's "STABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS" to conduct 56 batches of vaccine finished products. ℃ Accelerated thermal stability test. The three batches of vaccine combined vaccine finished products were stored at 56°C for 1 hour, 4 hours, 8 hours, 12 hours, 24 hours and 72 hours respectively, and the titers of measles virus and Japanese encephalitis virus in the combined vaccine were measured by sampling. The experimental results are shown in Table 2:
[0066] Table 2 Titers of measles JE vaccine after storage at 56°C for different time
[0067]
[0068] It can be seen from Table 2 that the combined live attenuated measles and Japanese encepha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com