JE vaccine soluble microneedle patch and preparation method thereof
A soluble, micro-needle sticking technology, applied in biochemical equipment and methods, microorganisms, pharmaceutical formulations, etc., can solve the problems of decreased mechanical strength of soluble micro-needles, and achieve the best immune enhancement effect, high mechanical strength of the needle body, immune good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The negative mold preparation of embodiment 1 Japanese encephalitis vaccine microneedles
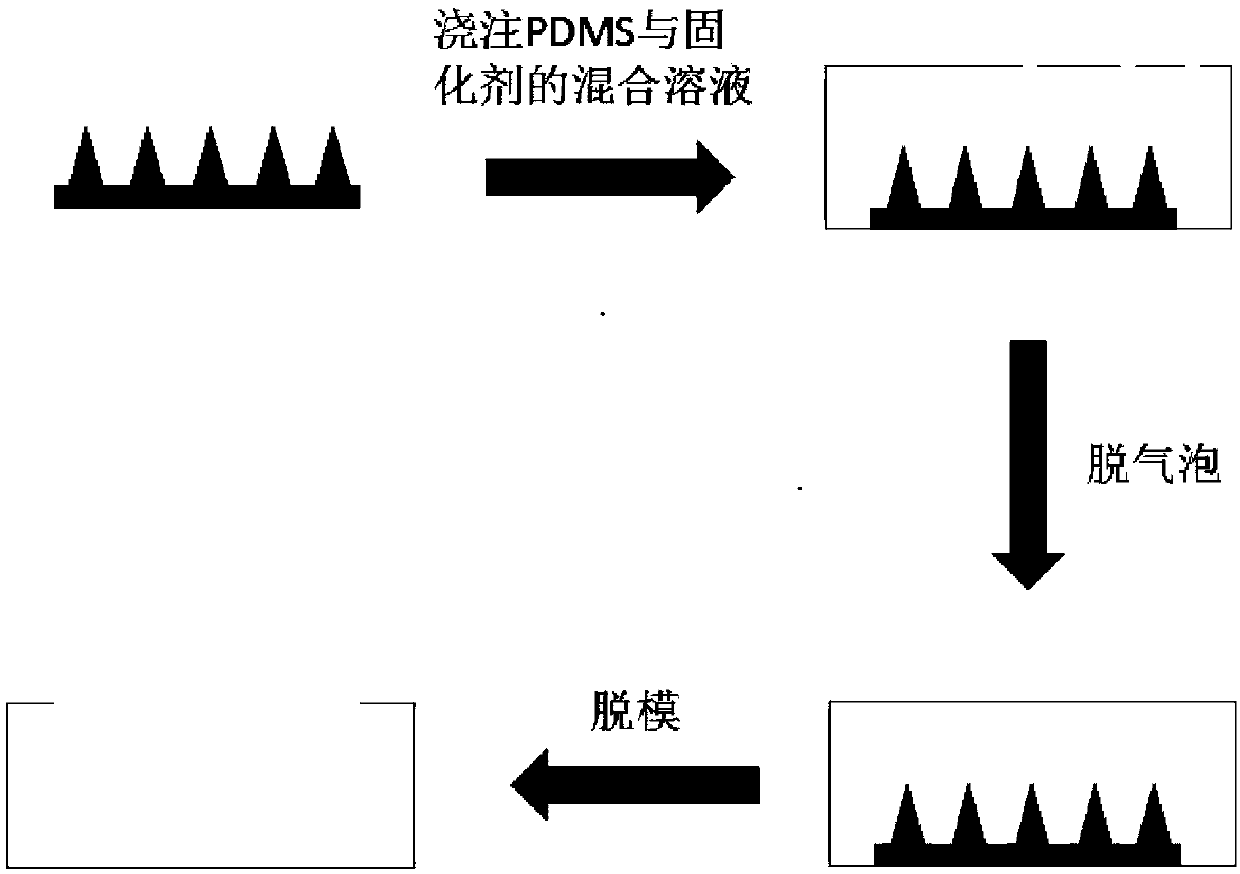
[0033] Such as figure 1 , the present invention adopts mold-inverting method to prepare the female mold of Japanese encephalitis vaccine microneedle, and the steps are as follows:
[0034] Place the metal male mold microneedle in the cuboid container with the needle tip facing upward. The polydimethoxysiloxane (PDMS) and the curing agent are configured according to the mass ratio of 10:1 and then poured into a rectangular parallelepiped container; the container is placed in a vacuum drying oven, and the vacuum degree of the parameters is respectively set to 0.07MPa, The time is 5 minutes to remove the air bubbles in the mixed solution; then put the container into an oven, set the temperature parameter to 50°C, take it out after 5-8 hours, and demould to obtain a PDMS microneedle female mold.
Embodiment 2
[0035] The preparation of embodiment 2 JE vaccine microneedles
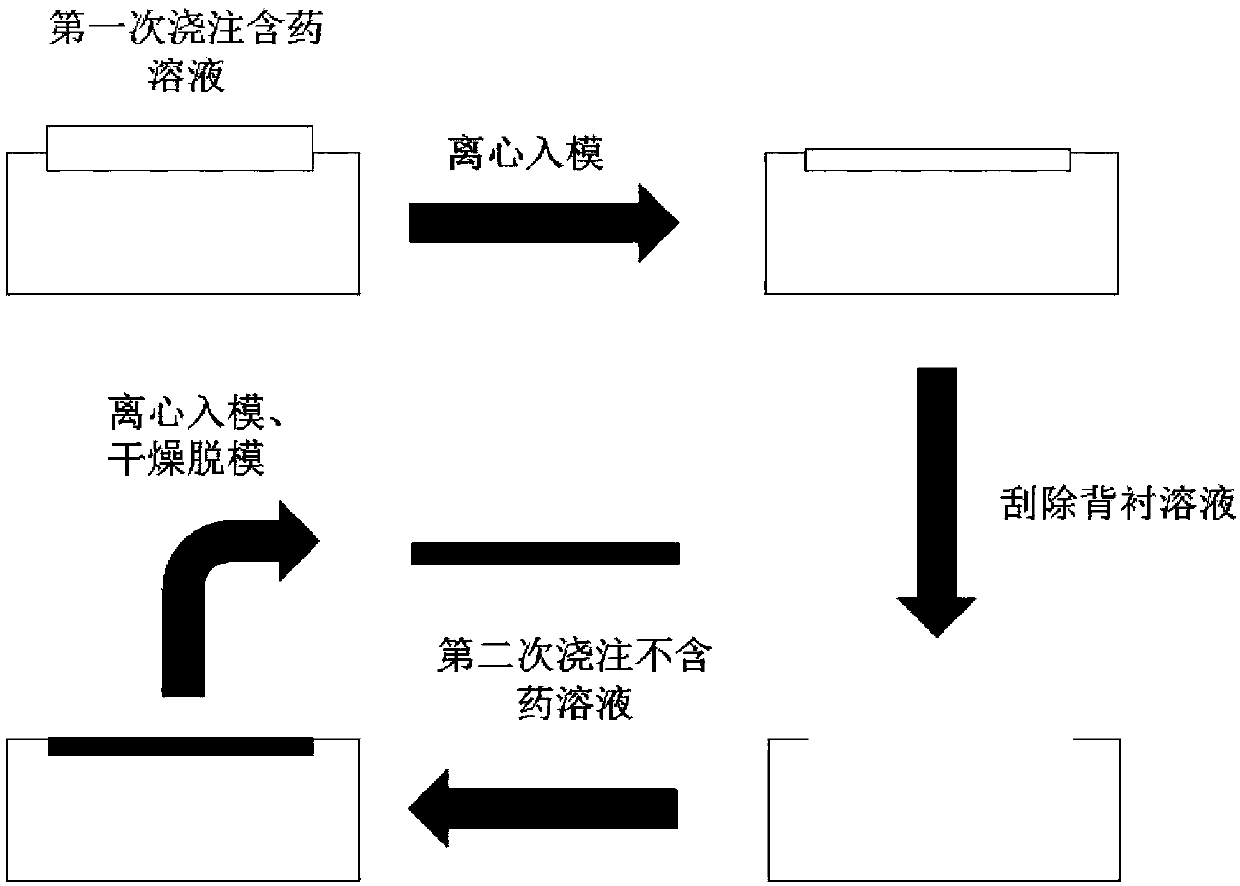
[0036] Such as figure 2 , the present invention adopts secondary centrifugation into mold method to prepare Japanese encephalitis vaccine microneedle, and the steps are as follows:
[0037] 1) After mixing the various ingredients in the prescription in proportion, dissolve it with an appropriate amount of solvent (deionized water) to form a uniform mixture (i.e. needle body fluid), take an appropriate amount of needle body fluid into a centrifuge tube, place it in a centrifugal precipitator, and centrifuge Remove the air bubbles in the needle body fluid and let it stand for later use.
[0038] 2) Take the needle body fluid obtained after centrifugation in step 1), pour it on the PDMS mold prepared in Embodiment 1 of the present invention, then place the PDMS mold in a desktop low-speed centrifuge, and centrifuge at a speed of 3000 rpm for 5 minutes, so that the needle body fluid is injected into the hole of the...
Embodiment 3
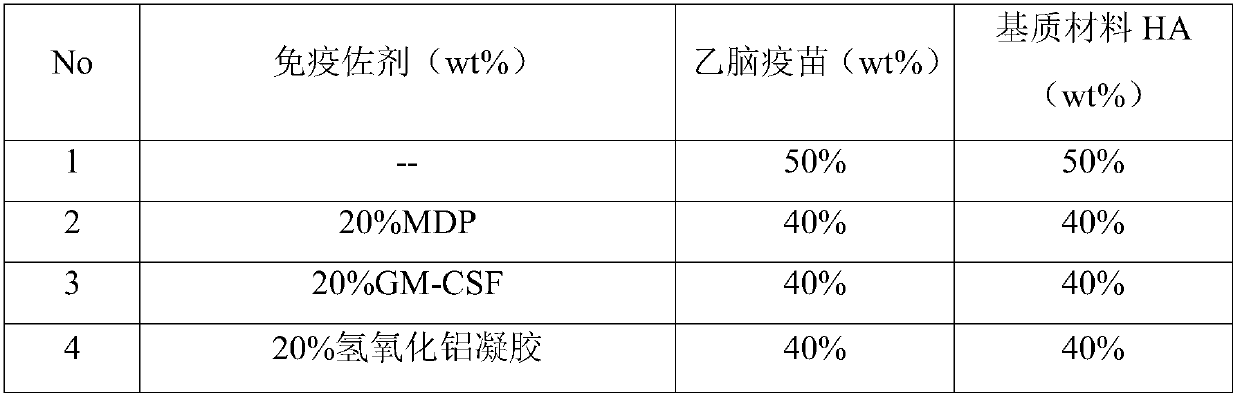
[0041] Example 3 Effect of the use of different immune adjuvants on the immune effect of Japanese encephalitis vaccine soluble microneedles
[0042] Assuming that the proportion of the immune adjuvant in the total mass of the needle is a certain value, only the components of the immune adjuvant are changed, and the influence of different combinations of the immune adjuvant as shown in Table 1 on the immune effect of the microneedle is investigated. This example For the related microneedle and negative mold, its preparation method refers to Example 1 and Example 2 of the present invention.
[0043] Table 1 Effect of different immune adjuvants on the immune effect of JE vaccine soluble microneedles
[0044]
[0045]
[0046] For 11 microneedle prescriptions in Table 1, after making soluble microneedles with reference to the method of embodiment 1 and embodiment 2 of the present invention, detect the immune effect of soluble microneedles as follows:
[0047] Eighteen solub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com