New application of rabies human immunoglobulin in preparing medicine for preventing and controlling Japanese encephalitis and combined vaccine for rabies and Japanese encephalitis
A technology of immunoglobulin and rabies vaccine, which is applied in the field of biological products, can solve problems such as the difficulty of universal vaccination and poor media control effect, and achieve the effect of scientific immunization procedures, good prevention effect and less vaccination frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Detection of JE antibody content in rabies patient immunoglobulin
[0037] Materials and Methods
[0038] 1. Immunoglobulin
[0039] 1.1 Cytomegalovirus human immunoglobulin: product of Massachusetts Public Health Biologic LaboratoriesUSA, Lot: MIVCMV-198.
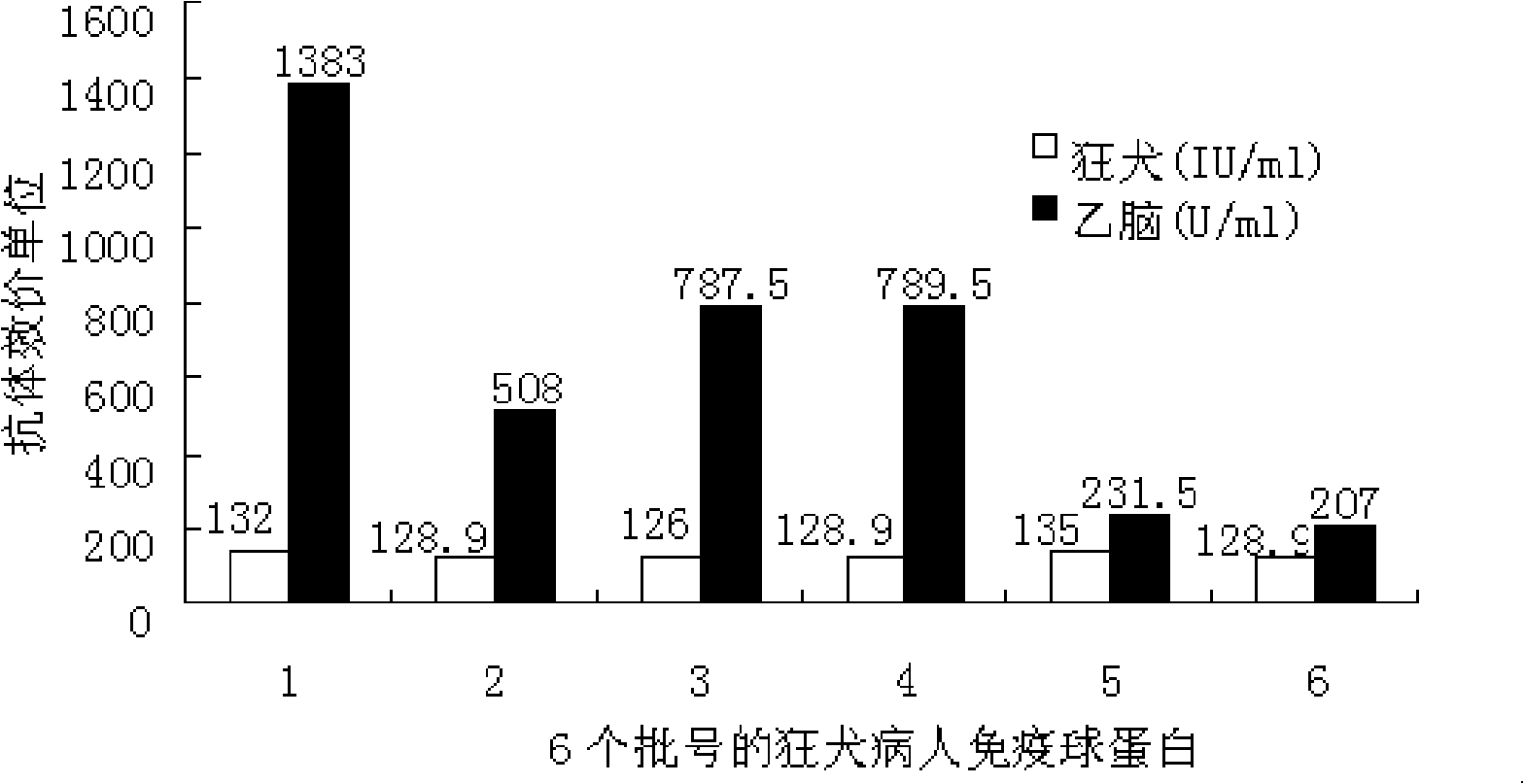
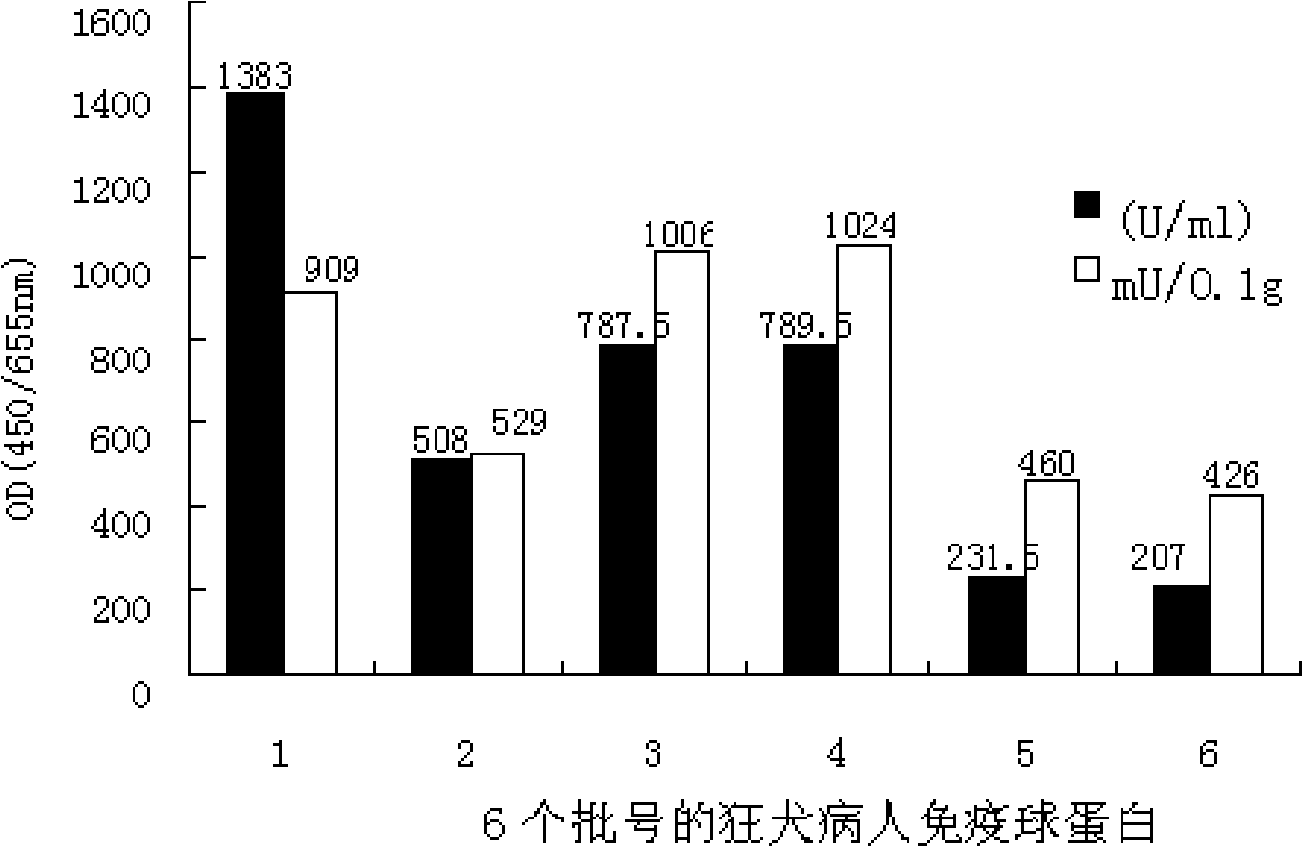
[0040] 1.2 Human immunoglobulin for rabies patients: 6 batches, the batch numbers are 200608002, 200605001,
[0041] 200704101, 200704201, 200802602, 200803403; protein content is 96.1, 152.1, 78.3, 77.1, 50.3, 48.6g / L, 2ml / bottle; rabies antibody titer is 128.9, 132, 126, 128.9, 135, 128.9IU / ml. The preparation process is to use purified rabies vaccine for human use (≥2.5IU / branch / person, product of Lanzhou Institute of Biological Products of the Ministry of Health or Shenyang Andy Biopharmaceutical Co., Ltd.), and the vaccine immunization procedure is carried out according to the instruction manual of purified rabies vaccine for human use. The plasma donors were given basic immunizations on days 0, ...
Embodiment 2
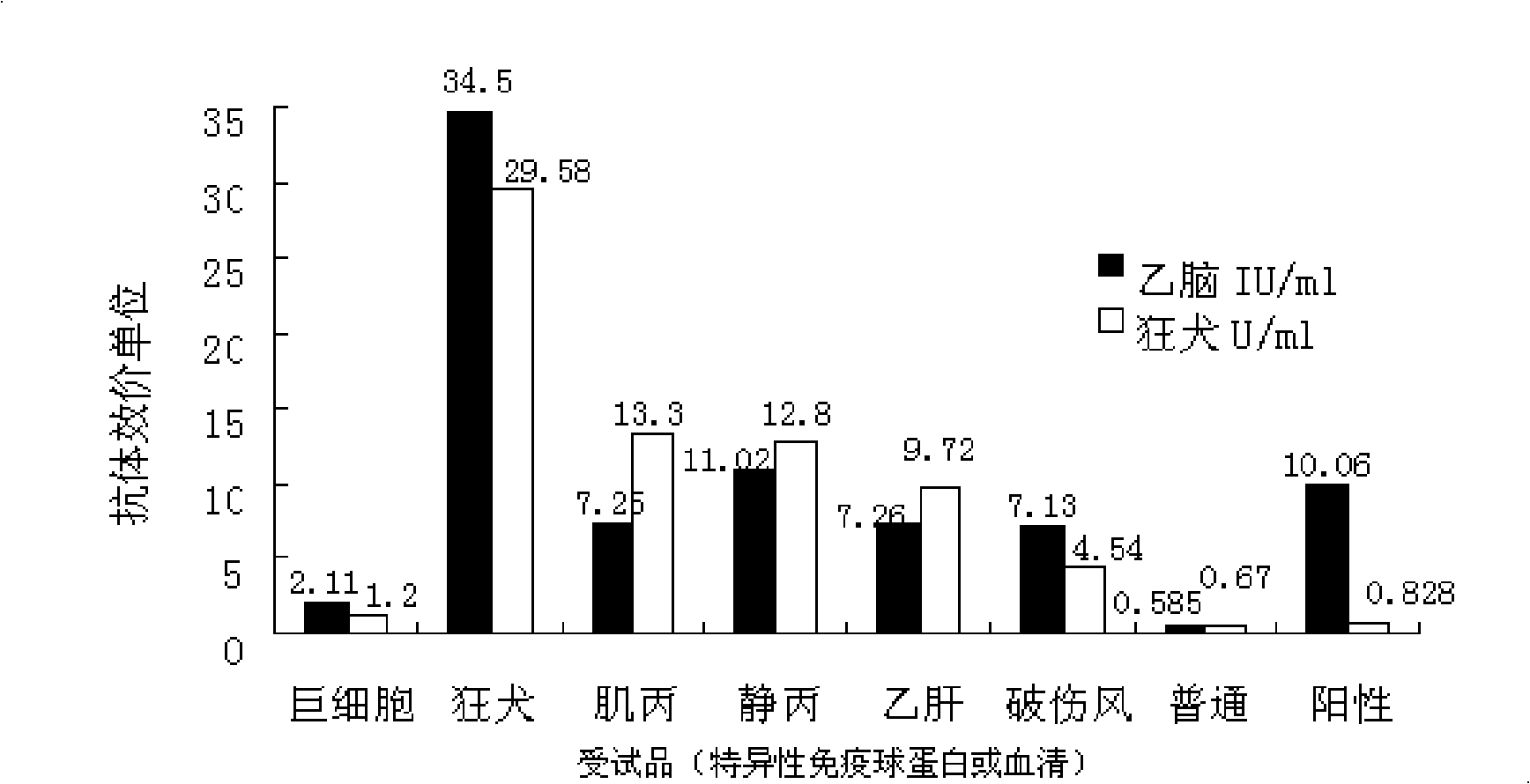
[0070] Comparison of Japanese Encephalitis Antibody in Embodiment 2 Rabies Immunoglobulin and Other Immunoglobulins
[0071] 1. Comparison of JE antibody titers and rabies antibody titers of various immunoglobulin test products
[0072] In order to investigate whether there is a reciprocal cross-antigen between JE and rabies virus, the JE and rabies antibody titers of the test products were compared. Compared with negative common serum, the rabies antibody titer of JE positive serum did not increase significantly. Therefore, the JE antibody titer in the immunoglobulin of rabies patients should not be caused by JE vaccine immunization (or latent infection). There is no relationship between JE antibody titer and rabies antibody titer found in other tested products.
[0073] 2. Comparison of rabies antibody titer and JE antibody titer of different batches of rabies patient immunoglobulin
[0074] In order to verify that there is a unidirectional cross-antigen between rabies an...
Embodiment 3
[0081] Example 3 Determination of Dosage of Preventing and Treating Japanese Encephalitis and Rabies Using Rabies Immunoglobulin
[0082] When using rabies patient immunoglobulin to prevent and treat Japanese encephalitis, the dosage and usage of the drug are calculated according to the titer of rabies antibody: the specification of rabies patient immunoglobulin is 100IU / ml (the potency of JE antibody is about 400±100U / ml ), 5-10IU / kg is used for emergency passive immunization prevention, and the total amount of medicine for a person with a body weight of 50kg is 250-500IU (including JE potency is about 1000-2000U). The titer will reach the level of 250-500U / L (calculated according to 4L serum / 50kg), and the half-life of IgG terminal elimination is 24.2 days, so a single dose should be used for emergency passive immunization prevention; for passive immunization therapy, it should be used in the early stage of the disease , taking into account the tolerance of intramuscular inj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com