Herpes simplex virus I-type gene recombinant attenuated live vaccine and preparation method thereof
A herpes simplex virus and attenuated live vaccine technology, applied in the field of biomedicine, can solve the problems of difficult to obtain pathogenicity, time-consuming and laborious, etc., and achieve the effects of difficult virulence recovery, reduction of morbidity, and control of recurrence and transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
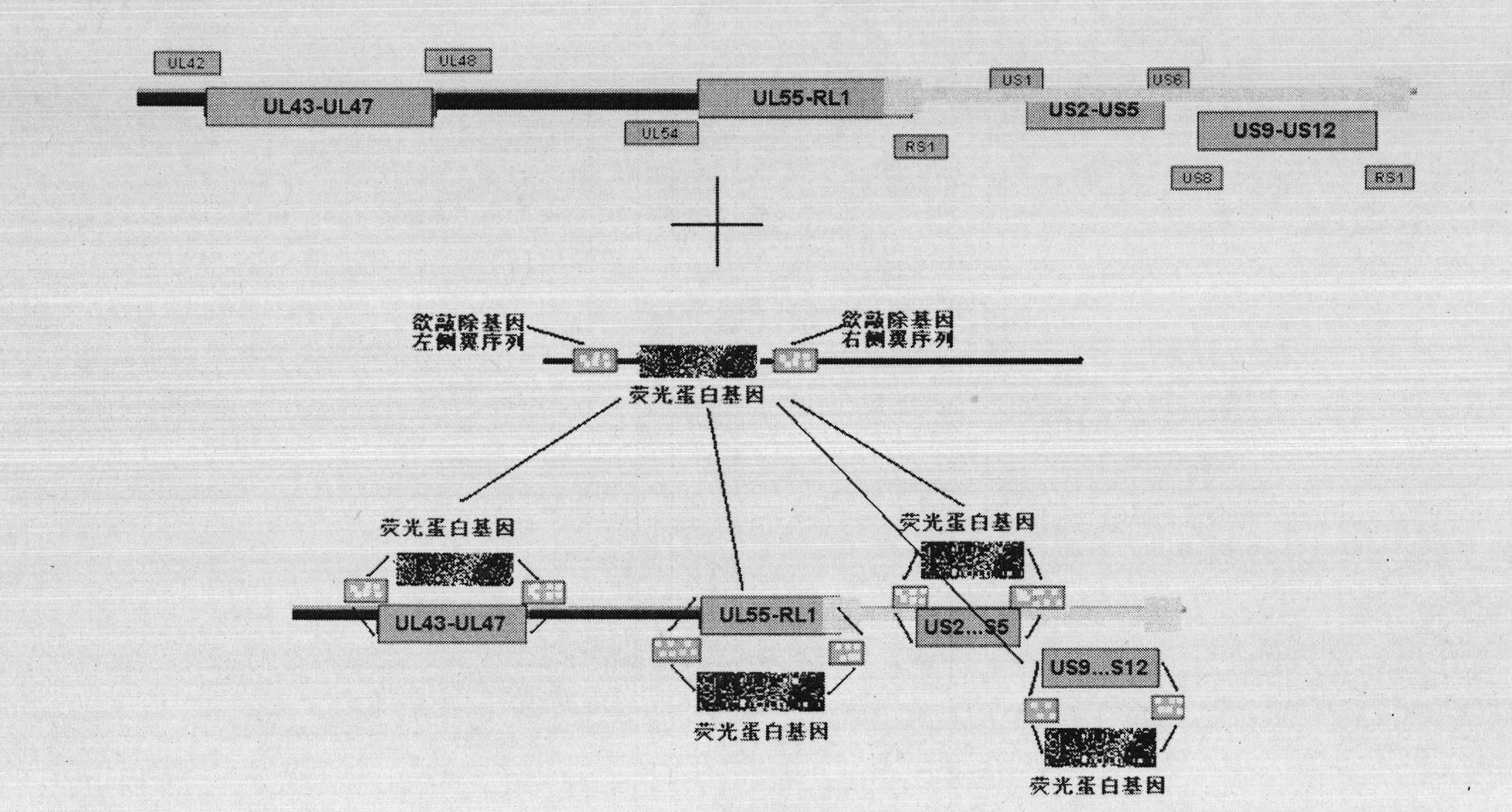
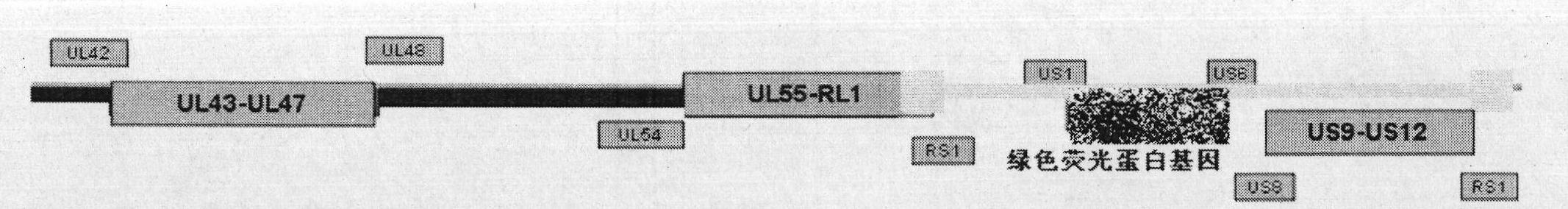
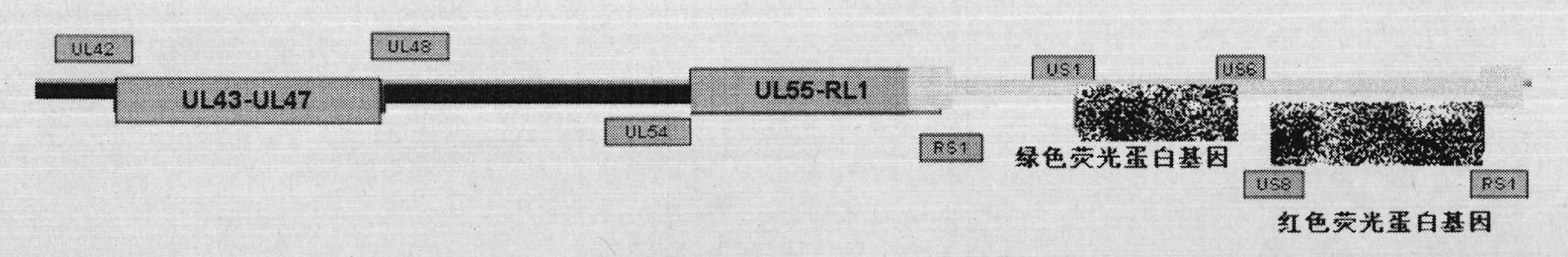
[0023] Embodiment 1 Construction of recombinant herpes simplex virus attenuated live vaccine JSH01S
[0024] 1. Construct the homologous recombination shuttle vector pShuttle-01S-GFP.
[0025] (1) Referring to the sequence of the US region US2-US5 of the HSV-1 genome with the accession number NC_001806 on NCBI, design the amplification primers for the left flank sequence of the US2 gene and the right flank sequence of the US5 gene, respectively.
[0026] (2) Primer design for US2 flanking sequences
[0027] A NotI site and three protective bases were added to the 5' end of the upstream primer of the US2 left flank sequence, and a PmeI, AseI site and three protective bases were added to the 5' end of the downstream primer of the US2 left flank sequence. After adding 30 cycles, the target band was recovered.
[0028] (3) Primer design for US5 flanking sequence
[0029] Add PmeI and MluI sites and three protective bases to the 5' end of the upstream primer of the US5 right fla...
Embodiment 2
[0040] Example 2 Replication and infection ability of recombinant herpes simplex virus attenuated live vaccine JSH01S is reduced
[0041] 1. Experimental materials: wild-type HSV-1, JSH01S, Vero cells, six-well plate.
[0042] 2. Experimental method: 5×10 5 Vero cells were cultured for 24 hours. According to the multiplicity of infection (MOI) of 0.1 and 1, four wells of the six-well plate were infected with wild-type HSV-1 and JSH01S respectively, and the remaining two wells were used as controls to observe the cytopathic effect of the virus-infected wells. Condition.
[0043] 3. Results: It is expected that wild-type HSV-1 will completely produce CPE in about 48 hours to 72 hours, while JSH01S can produce complete CPE in about 6 to 7 days, indicating that the recombinant strain JSH01S has the ability to replicate and infect compared with wild-type HSV-1 obviously decased.
Embodiment 3
[0044] The pathogenicity of embodiment 3 recombinant herpes simplex virus attenuated live vaccine JSH01S reduces
[0045] 1. Experimental method is identical with the method used in embodiment 2, gives control group wild-type herpes simplex virus type 1 20 microliters through nasal cavity infusion route, and normal dose group gives 20 microliters of JSH01S, and high dose group gives 100 microliters of JSH01S, observes mouse condition.
[0046] 2. Results: It is expected that the mice in the control group will begin to die on the 5th day after nasal instillation, and all die after the 13th day, and the mortality rate is 100%; the mice in the normal dose group and the high dose group are all alive, and the mortality rate is 0, It shows that compared with the wild type, JSH01S has almost no pathogenicity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com