Process for purifying human retinol binding protein and preparation process of polyclonal antibody thereof
A polyclonal antibody and protein-binding technology, applied in the field of medical immunity, can solve the problems of complex treatment of prealbumin antibody, difficult industrialization, and unsatisfactory extraction effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
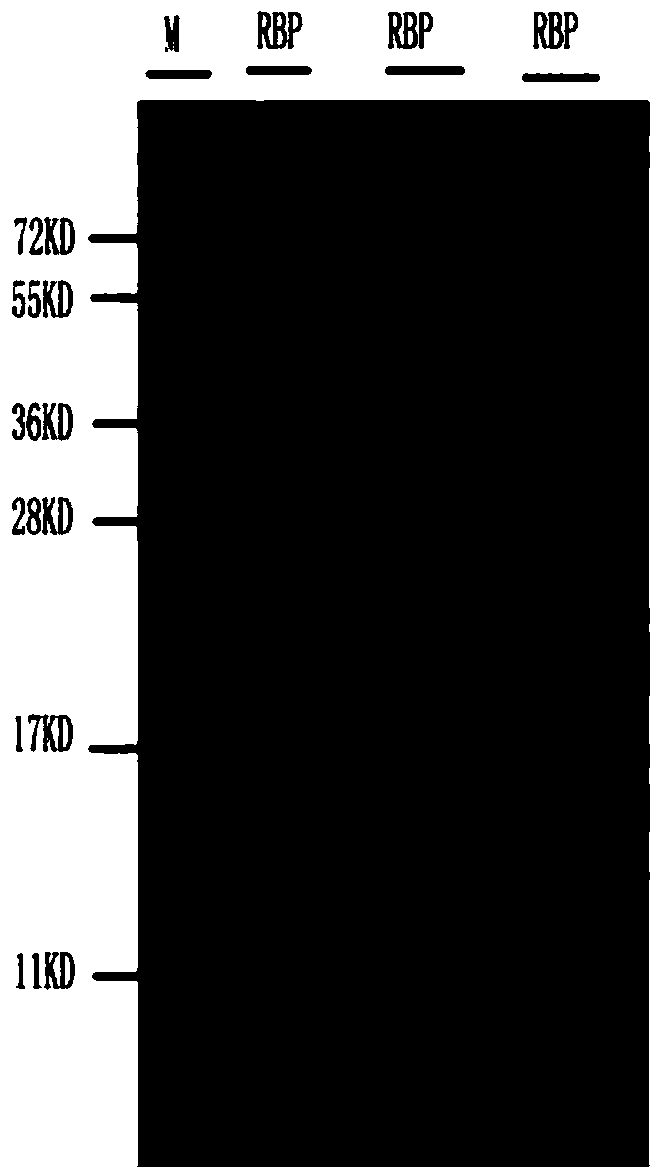
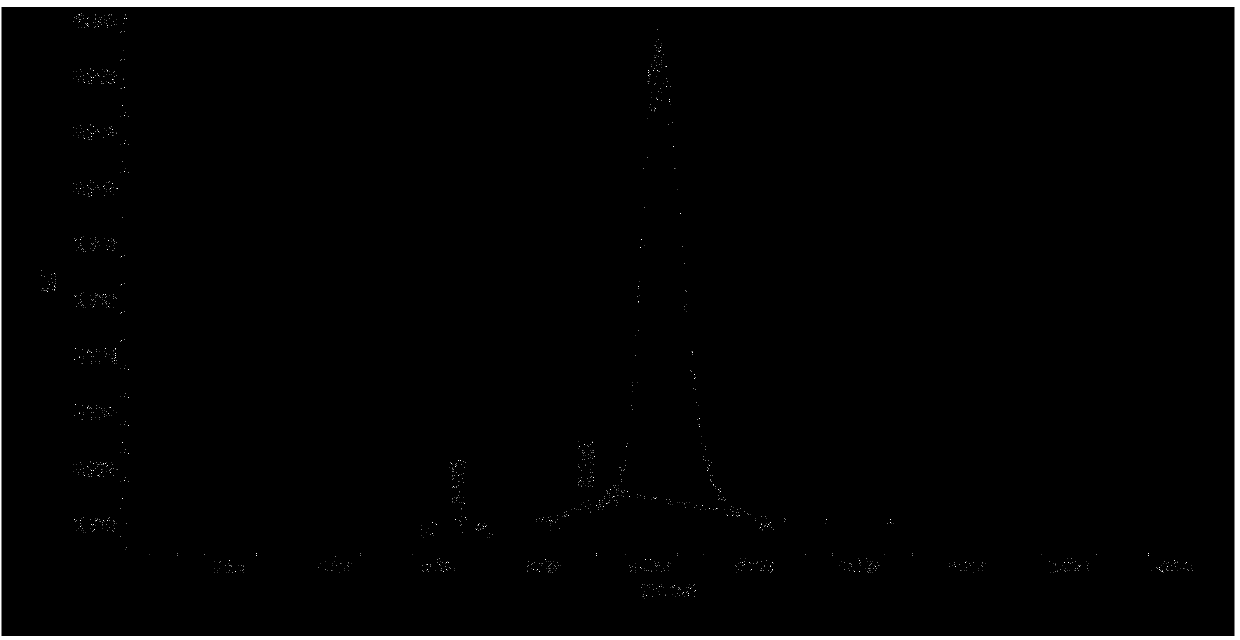
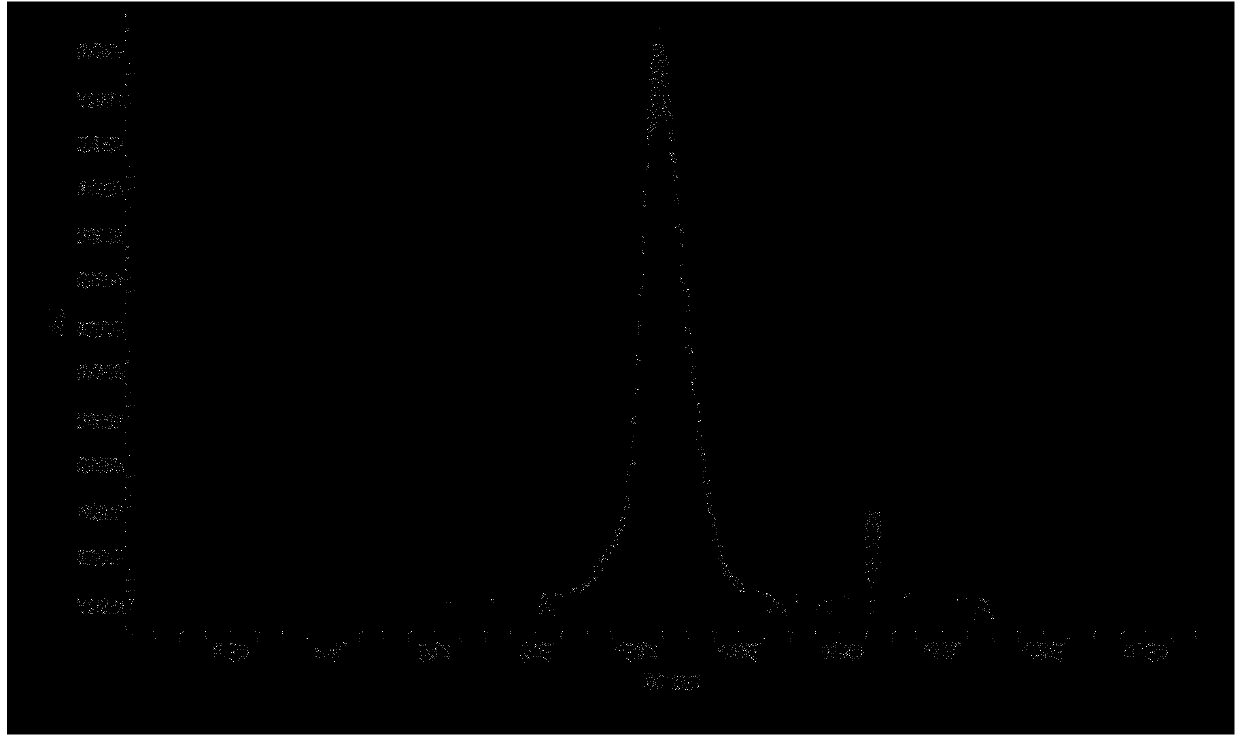
[0044] Example 1 Purification of Immunogen (RBP Antigen)
[0045] 1. Instruments and columns used:
[0046] 1.1 Instruments: simple protein purification system (including constant flow pump, UV detector, recorder) (Amersham Pharmacia Biotech Inc), GradiFrac TM Programming (Amersham Pharmacia Biotech Inc), Waters600 high-phase liquid chromatography (Waters, USA), etc. .
[0047] 1.2 Column: ion exchange column DEAE-Sepharose Fast Flow (DEAE-S), molecular sieve (Superdex 75, Sephacryls-200), hydrophobic column Phenyl Sepharose TM High Performance (PSHP) and CNBr-activated Sepharose 4B are produced by GE Healthcare; Protein Pak Glass 300SW is produced by waters.
[0048] 2. Pretreatment of urine in patients with renal impairment
[0049] 2.1 Centrifuge the patient's urine for 30 minutes (4000r / min), and take the supernatant;
[0050] 2.2 Add solid SAS (ammonium sulfate) to the supernatant to make the saturation reach 60%, and let stand overnight;
[0051] 2.3 Centrifuge the...
Embodiment 2
[0083] Example 2 Purification of Immunogen (RBP Antigen)
[0084] 1. The pretreatment of urine from patients with renal impairment is the same as in Example 1
[0085] 2. Separation and purification:
[0086] 2.1 Preparation of mobile phase:
[0087] Buffer A: 25mmol / L Tris pH=7.5+0.01%NaN 3 +1mM EDTA;
[0088] Measure 312.5mL 200mmol / L Tris pH=7.5, 1.25mL 20%aN 3 , 5mL 500mM EDTA mixed, add ultrapure water to 2.5L, mix well, that is.
[0089] Buffer B1: 20mmol / L Tris pH=8.0+50mmol / L NaCl+0.03%NaN 3 +1mM EDTA;
[0090] Measure 250mL 200mmol / L Tris pH=8.0, 31.25mL 4mol / L NaCl, 3.75mL 20%NaN 3 , 5mL 500mM EDTA mixed, add ultrapure water to 2.5L, mix well, that is.
[0091] Buffer B2: 15mmol / L Tris pH=8.0+250mmol / L NaCl+0.01%NaN 3 +1mM EDTA;
[0092] Measure 250mL 187.5mmol / L Tris pH=8.0, 156.25mL 4mol / L NaCl, 1.25mL 20%NaN 3 , 5mL 500mM EDTA mixed, add ultrapure water to 2.5L, mix well, that is.
[0093] Buffer C: 20mmol / L Tris pH=8.0+120mmol / L NaCl+0.03%NaN 3 +1mM ...
Embodiment 3
[0114] Example 3 Preparation of goat anti-human RBP polyclonal antibody
[0115] 1. Immunization of goats: Boer goats were selected for subcutaneous immunization (implementation of RBP antigen prepared in 1+Freund's adjuvant) injection.
[0116]2. Collect goat antiserum: collect goat antiserum and perform immunoelectrophoresis on the antiserum. If there are bands of immunoglobulins and albumin, remove the collected antiserum by CNBr-activated Sepharose 4B column coupled with normal human serum. Impurity bands of immunoglobulins and albumin are obtained to obtain goat antiserum after removal of impurities.
[0117] 3. Purification of the goat anti-human RBP polyclonal antibody: Precipitate the goat anti-human ascites globulin in the goat antiserum after the impurity removal with ammonium sulfate solution with a saturation of 50%, centrifuge at 8000r / min for 30min, take the precipitate and dialyze it for desalination. Purified by DEAE-Sepharose Fast Flow (DEAE-S) ion exchange c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com