Mycoplasma hyopneumoniae subunit vaccine and preparation method and application thereof
A technology for mycoplasma hyopneumoniae and subunit vaccines, applied in biochemical equipment and methods, vaccines, veterinary vaccines, etc., can solve the problems of inability to secrete and express, high production costs, and difficult control of production quality, so as to shorten the growth cycle and reduce Production cost, the effect of enhancing the protective effect of mucosal immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Construction of the mycoplasma antigen chimeric protein expression vector based on the Bacillus megaterium expression system
[0049] (1) Expression vector construction and identification
[0050] 1. Acquisition of Escherichia coli heat-labile enterotoxin LTB gene
[0051] Referring to the Escherichia coli LTB gene sequence (WP_012846869.1), a 390bp LTB gene sequence was artificially synthesized according to the codon preference of Bacillus megaterium.
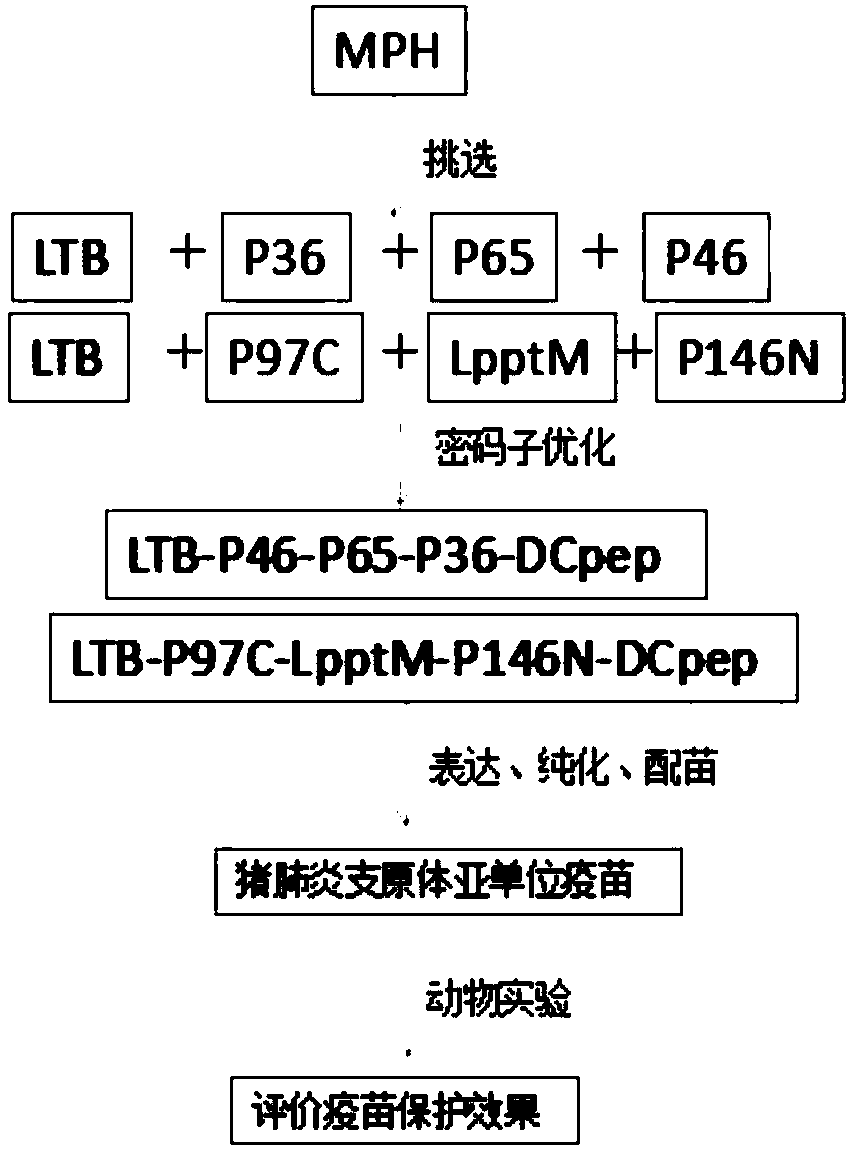
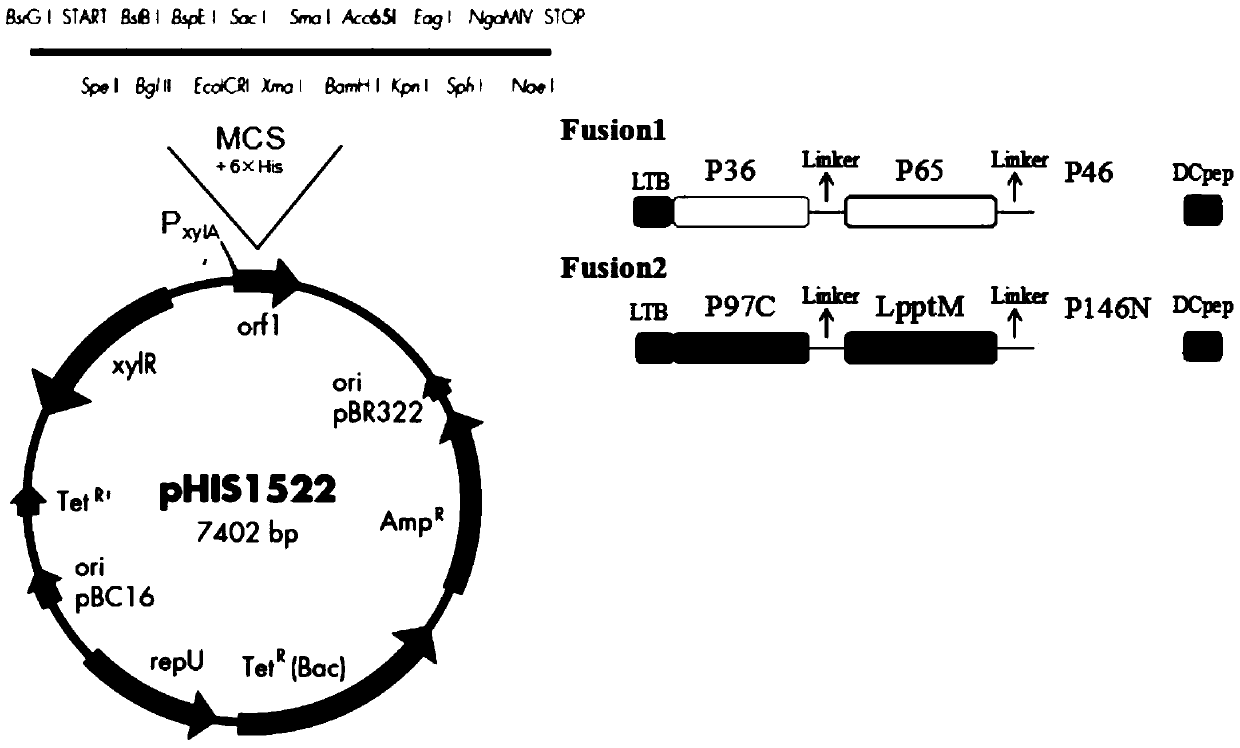
[0052] 2. Acquisition of mycoplasma chimeric protein LTB-P36-P65-P46-Dcpep and LTB-P97-LpptM-P146-Dcpep genes and vector construction.
[0053] Referring to the genome sequence of Mhp (J strain NC_007295.1), according to the amino acid sequences of immunogenic proteins such as P36, P46, P65, P97C, P146N, LpptM, etc., LTB-P36-P65 was artificially synthesized according to the codon preference of Bacillus megaterium - the gene of P46-Dcpep and LTB-P97-LpptM-P146-Dcpep chimeric protein, and corresponding enzyme ...
Embodiment 2
[0068] Example 2 Preparation and animal experiments of chimeric protein fusion1 and fusion2 subunit vaccines
[0069] 1. Subunit vaccine prepared by chimeric protein fusion1 and fusion2 of the present invention
[0070] The concentration of purified fusion1 and fusion2 was measured with Montanide TM Gel01 adjuvant (purchased from SEPPIC) was emulsified to prepare a subunit vaccine with a protein concentration of 150 micrograms per milliliter, used in the following examples, and stored at 4°C.
[0071] 2. Inspection of technical indicators of subunit vaccine prepared by chimeric protein fusion1 and fusion2 of the present invention
[0072] (1) Physical properties
[0073] Appearance This product is milky white homogeneous emulsion. The dosage form is a one-way dosage form (oil-in-water). Take a clean straw to suck a small amount of vaccine and drop it in cold water, both on the water surface and in the water. Viscosity Use a pipette with an outlet diameter of 1.2mm to draw...
Embodiment 3
[0096] Example 3 The protective effect of subunits prepared by chimeric proteins fusion1 and fusion2 against viruses
[0097] 1. Preparation and immunization of fusion1 and fusion2 subunit vaccines
[0098] Add fusion1 and fusion2 prepared in Example 1 in turn into a sterile beaker. Then add 38ml of PBS solution with a pH of 7.2, and finally add 10ml of Gel01 adjuvant (produced by SEPPIC, France), so that the contents of the two antigenic proteins are respectively 150μg / ml, stir at 37°C and 500rpm for 10 minutes, and the vaccine is obtained. 100ml.
[0099] See Table 2 for the status of immunization and challenge.
[0100] Table 2 Vaccine immunization and challenge groups
[0101]
[0102]
[0103] 2. Results of Mycoplasma hyopneumoniae challenge
[0104] Table 3 shows the results of lung lesion index after M. hyopneumoniae challenge. The results showed that the average lung lesion index of vaccine 1 and vaccine 2 were 4.0 and 4.2 respectively, and the lung lesion o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com