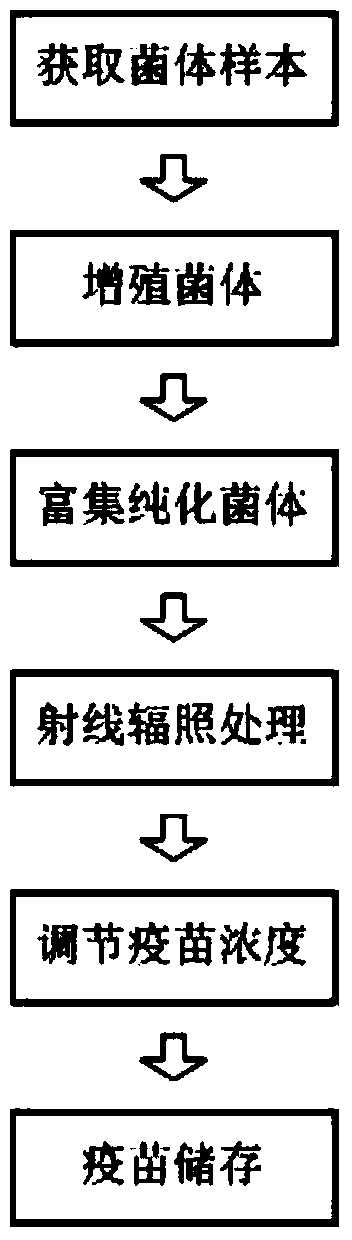
Pseudomonas aeruginosa vaccine and preparation method thereof
A Pseudomonas aeruginosa and vaccine technology, applied in biochemical equipment and methods, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of high toxicity of the body, low vaccine protection effect, difficult to cure infection, etc., and achieve enhanced immune response Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1, preparation and efficacy test of Pseudomonas aeruginosa vaccine of the present invention
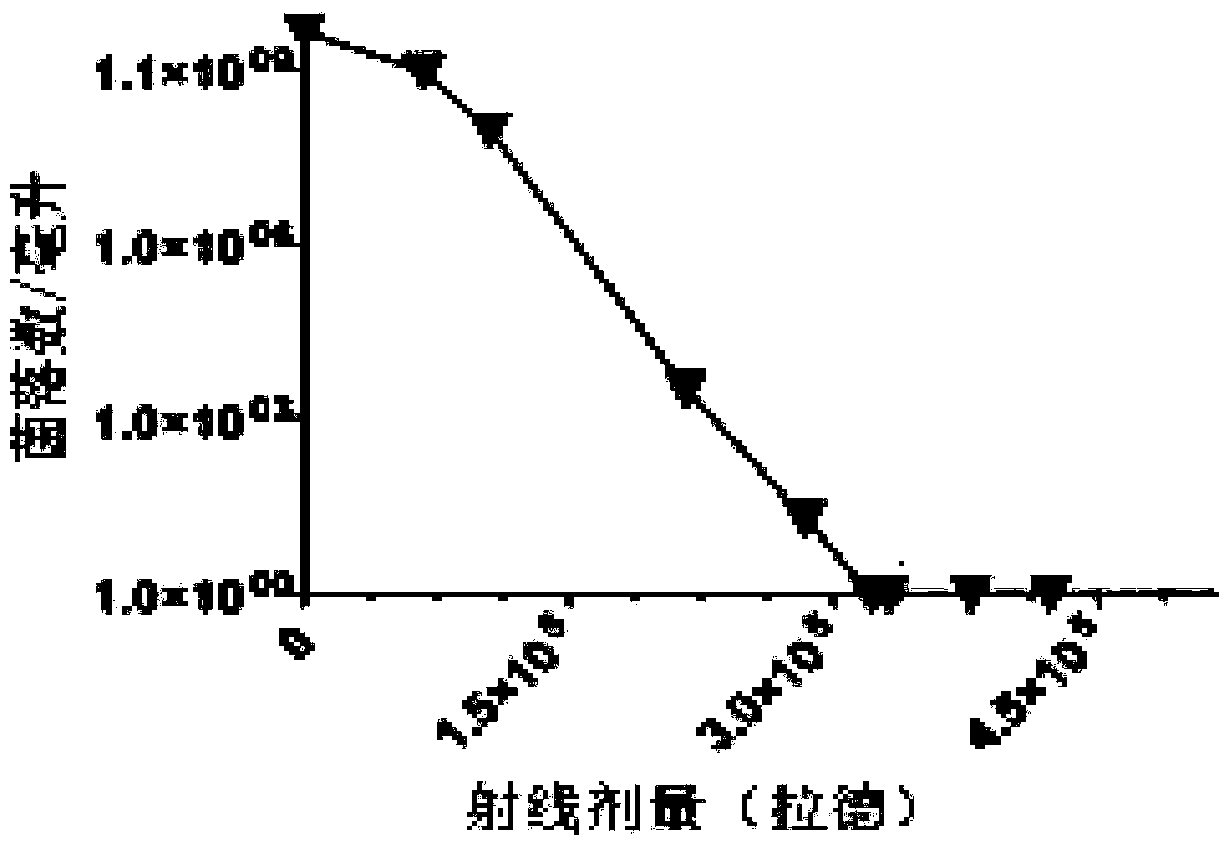
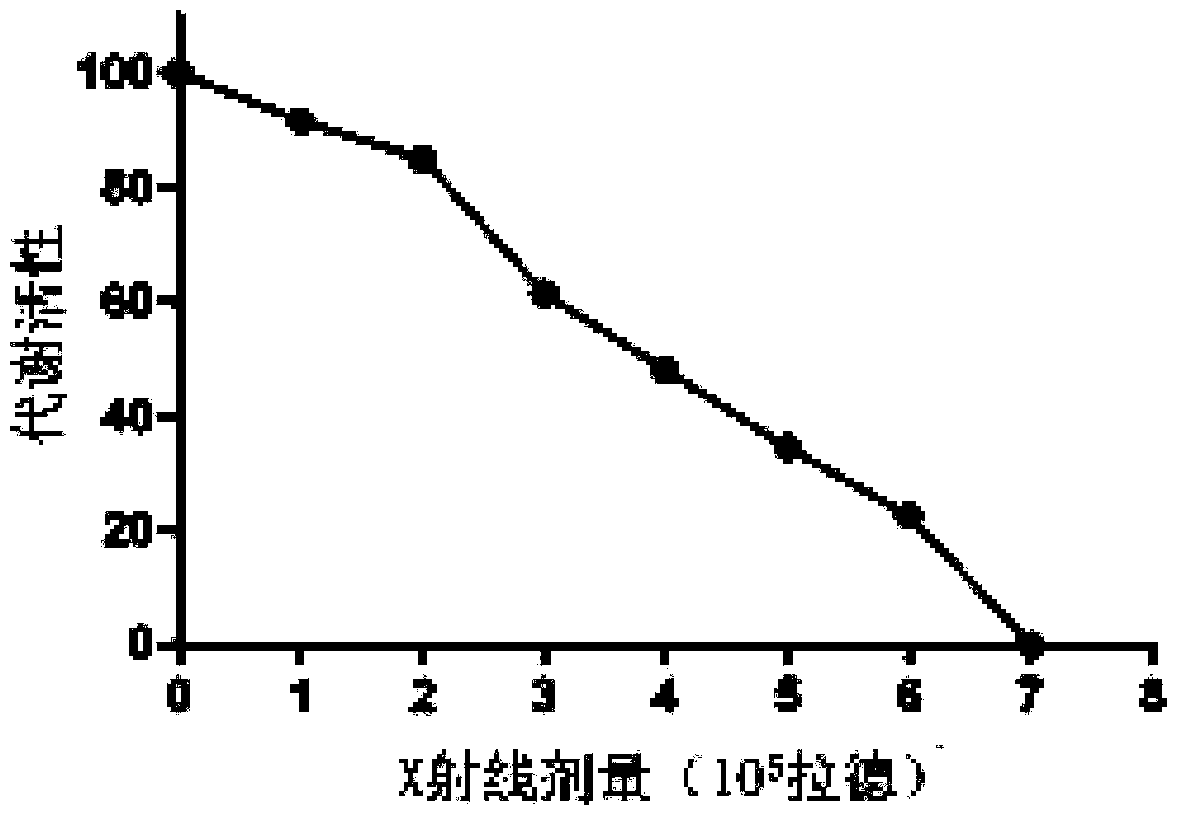
[0068] Select the standard strain of Pseudomonas aeruginosa ATCC 27853, use LB medium to culture and proliferate on a bacterial shaker at 37°C and 220r / min for 16 hours, and use 0.5 McFarland turbidity to detect the concentration, so that the bacteria reach about 10 11 number of bacteria. Collect the bacterial culture medium, centrifuge at 3500r / min for 10min, collect the centrifuged sediment and resuspend it with sterile saline, then centrifuge again according to the above conditions, and repeat 3 times. Resuspend the bacterial centrifuged sediment with sterile normal saline, put it in a 50ml BD tube, place it under the center of the radioactive source of the X-ray irradiator for irradiation, and dilute the irradiated bacteria with sterile normal saline for 100 After doubling, spread evenly on the LB agar plate with a bacteria-coating stick, and incubate at 37°C fo...
Embodiment 2
[0071] Embodiment two, preparation and efficacy test of Pseudomonas aeruginosa vaccine of the present invention
[0072] Select the standard strain of Pseudomonas aeruginosa ATCC27853, use LB medium to culture and proliferate on a bacterial shaker at 37°C and 220r / min for 16 hours, and use 0.5 McFarland turbidity to detect the concentration, so that the bacteria reach about 10 11 number of bacteria. Collect the bacterial culture medium, centrifuge at 3500r / min for 10min, collect the centrifuged sediment and resuspend it with sterile saline, then centrifuge again according to the above conditions, and repeat 3 times. After resuspending the bacterial centrifuged sediment with sterile physiological saline, put it in a 50ml BD tube, place it under the center of the radioactive source of the X-ray irradiator, and irradiate it under the condition of 8Gy / min for a total of 470 minutes of intermittent irradiation, (20 Minutes, stop after 5 minutes and continue, repeat the above cycle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com