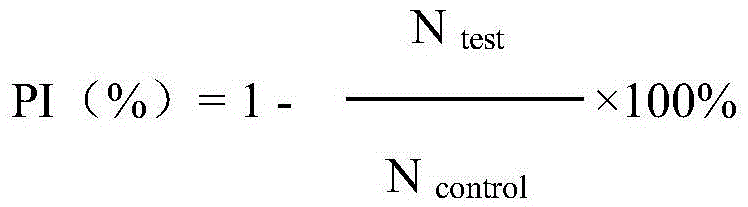Fusion protein with anti-tumor, anti-inflammation and oculopathy-treatment functions and preparation method and application thereof
A fusion protein and ophthalmic disease technology, which is applied in the direction of antineoplastic drugs, chemical instruments and methods, and medical preparations containing active ingredients, etc., can solve the problems of short half-life, single target, and high cost of chemical synthesis, and achieve low toxicity, Enhance the stability and improve the efficacy of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Construction of the carrier
[0047] The full lengths of the target genes of the two fusion proteins are 195bp and 174bp respectively, the plasmid vector is pGEX-4T-1, the cloning site is BamHI / XhoI, and the host bacteria are DH5α or CHO cells. Among them, GGATCC is a BamHI restriction site, CTCGAG is an XhoI restriction site, and TAGTAA is two stop codons.
[0048] Target gene base sequence:
[0049] The base sequence of protein A gene is:
[0050] 5' GGATCC GCATGCGATTGCCGTGGTGATTGCTTTTGCGGTGGTGGTGGTATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGCGGAAGCCGCGGCGAAAGAAGCCGCGGCGAAAGAAGCCGCGGCGAAAGAAGCCGCGGCGAAAGCGATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGGTGGTGGTGGTCGTGGTGAT TAGTAACTCGAG 3'
[0051] The gene base sequence of protein G is:
[0052] 5' GGATCC GCATGCGATTGCCGTGGTGATTGCTTTTGCGGTGGTGGTGGTATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGGTGGTGGTGGTTCTGGTGGTGGTGGTTCTGGTGGTGGTGGTTCTATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGGTGGTGGTGGTCGTGGTGAT TAGTAACTCGAG 3'
[0053] (2) Expression of the targ...
Embodiment 2
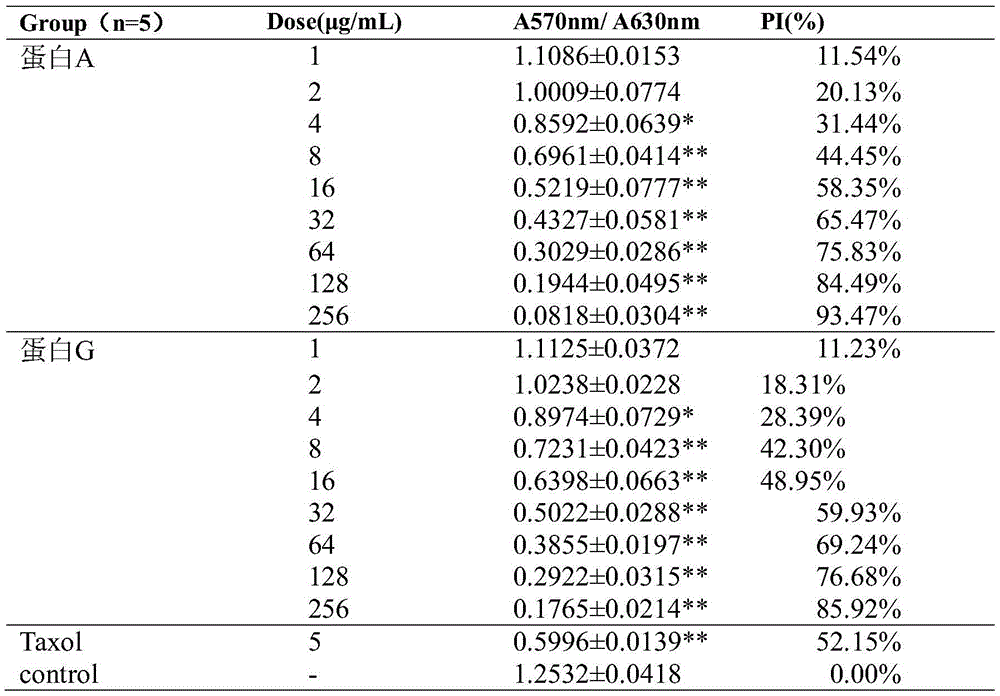
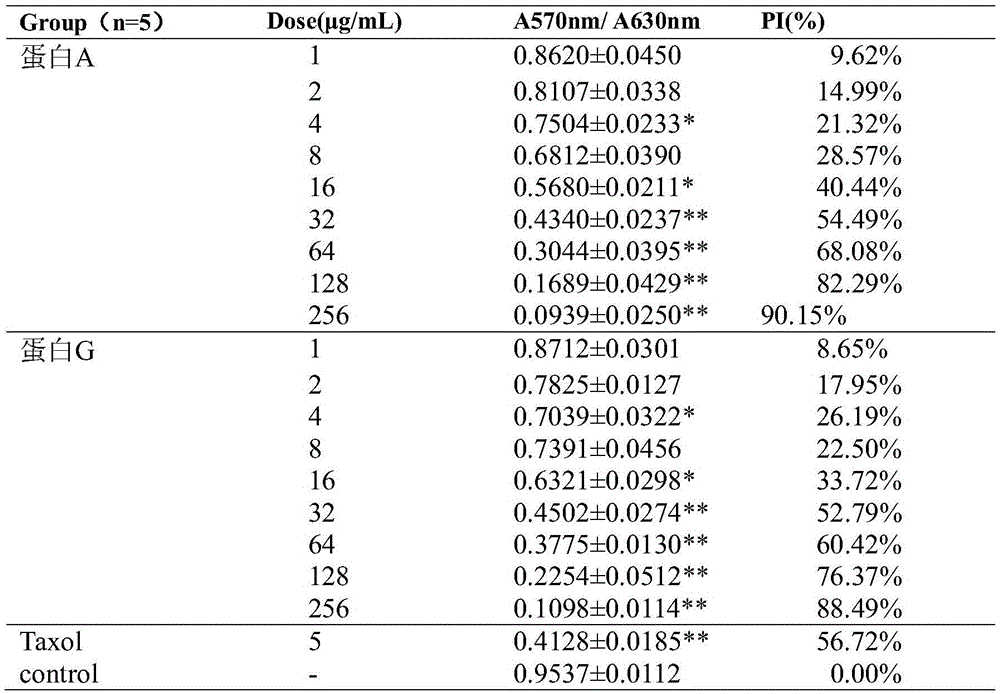
[0081] Inhibitory Effects of Fusion Proteins on the Proliferation of Various Tumor Cells
[0082] MTT method was used to detect the inhibitory effect of the integrin blocker fusion protein obtained in Example 1 on the proliferation of various tumor cells, including melanoma cell B16F10, gastric cancer cell MGC-803, lung cancer cell A549, liver cancer cell Hep-G2, Breast cancer cell MDA-MB-231, colon cancer cell HCT-116, human glioma U87, cervical cancer cell Hela.
[0083] Tumor cells were incubated at 37°C, 5% CO 2 When cultured in an incubator with a density of more than 90%, it was digested with trypsin and collected, and the cells were resuspended in culture medium and counted under a microscope, and the cell concentration was adjusted to 3.0×10 4 cells / mL, seed the cell suspension into a 96-well plate, 100 μL per well, and inoculate at 37°C, 5% CO 2 Incubate overnight in the incubator. Dilute fusion protein A, fusion protein G, and positive drug Taxol to respective pre...
Embodiment 3
[0124] Three-dimensional transwell method to detect the activity of fusion protein A and protein G in inhibiting the migration of human umbilical vein endothelial cells
[0125] Human umbilical vein endothelial cells (HUVEC) were incubated with endothelial cell culture medium containing 5% fetal bovine serum and 1×ECGS at 37°C, 5% CO 2 When cultured in the incubator to a confluence of more than 90%, the transwell method was used to detect the activity of fusion protein A and protein G in inhibiting the migration of endothelial cells. The endothelial cell HUVEC only used the 2nd to 8th passages. The specific operation is as follows:
[0126] (1) Dilute 10mg / mL Matrigel with DMEM medium at a rate of 1:4, spread it on the transwell chamber membrane, and air-dry at room temperature;
[0127] (2) Digest the HUVEC cells cultivated to the logarithmic growth phase with 0.2% EDTA, collect, wash twice with PBS, resuspend with endothelial cell culture medium containing 0.1% BSA, count un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com