DNA vaccine for preventing toxoplasmosis of humans or animals
A DNA vaccine and toxoplasmosis technology, applied in the field of vaccines, can solve the problems of different immune protection effects, the ROP2DNA vaccine immune protection effect cannot reach a satisfactory level, and the ROP is less, so as to improve the immune protection response and survival rate. , Improve the immune response and survival rate, reduce the effect of the formation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Example 1. Toxoplasma gondii ROP19 epitope prediction
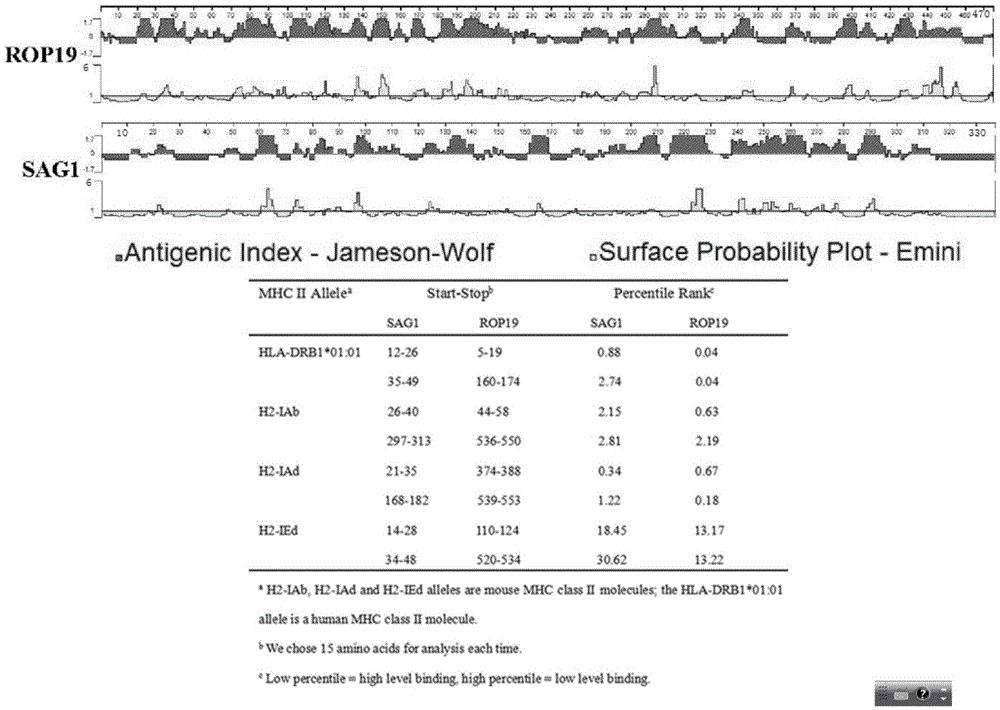
[0033] Predict the B cell dominant epitope of ROP19 (ToxoDB10.0(http: / / toxodb.org / toxo / )(GeneID:TGME49_242240)) by comprehensive analysis of DNAStar-Protean software; use IEDB to predict both human and BALB / C mice Toxoplasma gondii ROP19 T cell dominant epitope restricted by MHC molecules ( figure 2 ).
example 2
[0034] Example 2. DNA vaccine of recombinant Toxoplasma gondii ROP19 gene
[0035] According to the gene sequence of Toxoplasma gondii ROP19, the synthetic primers were designed as follows: upstream primers:
[0036] 5'-CGGGGTACCATGAGAAGGCTGCTGCTTTC-3', as shown in SEQ ID NO: 1; downstream primer:
[0037] 5'-CGGGATCCTCACTGAGATCTGGATGC-3', as shown in SEQ ID NO:2.
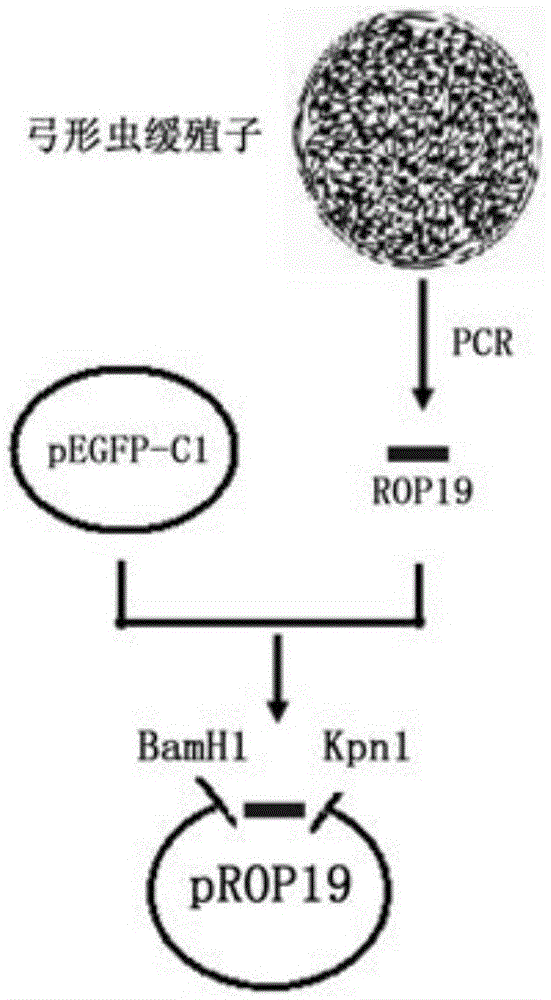
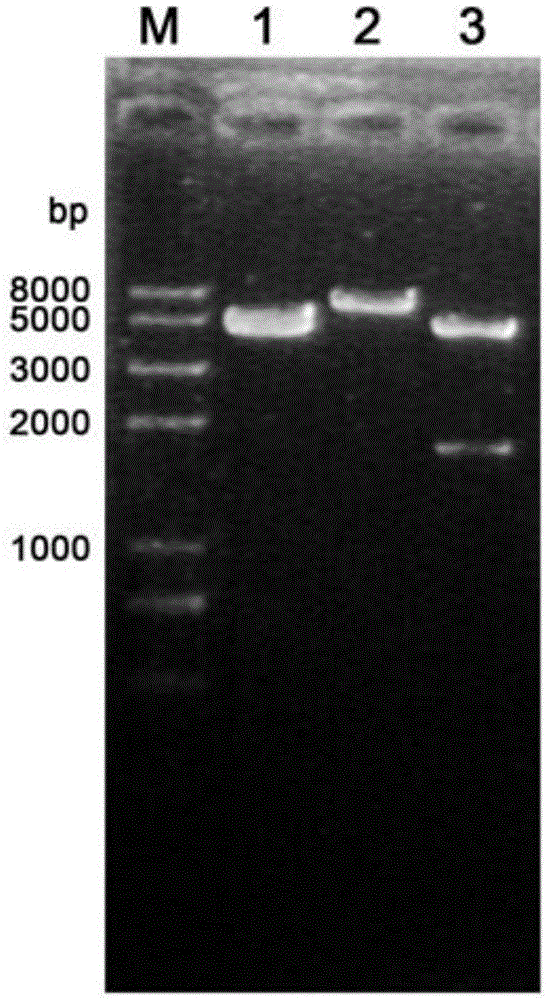
[0038] Such as figure 1 As shown, the extracted Toxoplasma gondii genomic DNA was used as a template to amplify the ROP19 gene, and the reaction conditions were: denaturation at 95°C for 30s, annealing at 55°C for 30s, extension at 72°C for 3min, a total of 30 cycles. The amplified product was subjected to 1% agarose gel electrophoresis, and the gel was cut to recover the target fragment. The recovered PCR product and the pEGFP-C1 plasmid vector were respectively digested for 3 hours and then electrophoresed. After cutting the gel, the kit was used to recover the digested PCR product The purified product and th...
example 3
[0039] Example 3. Toxoplasma gondii recombinant gene vaccine immunized BALB / c mice
[0040] SPF grade female BALB / c mice (6-8 weeks) were purchased from the Experimental Animal Center of Shandong University. Forty-five mice were randomly divided into 3 groups. The mice in the experimental group were injected with 100 μg of the recombinant vaccine pEGFP-C1-ROP19 through the hind leg muscles, and the mice in the control group were injected with 100 μg of the empty vector pEGFP-C1 and 100 μg of PBS. Mice were immunized three times with two weeks apart.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com