Preparation of infectious bursal disease (IBD) protein engineering vaccine
A capsule protein and vaccine technology, applied in the field of biotechnology genetic engineering, can solve the problems of subclinical secondary infection, mutant strains, high virulence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Design Idea of Bursal Protein Engineering Vaccine Protein
[0023] According to the amino acid sequences of the structural proteins VP2 and VP3 of the main prevailing strains of Bursa Fabricius at present, the present invention uses relevant bioinformatics software DNASTAR, BIMAS and SYFPEITHI to analyze the B cell neutralizing epitopes and T cell epitopes of the prevailing strains, and introduces poultry The cytokine interleukin 15 (IL-15) acts as an adjuvant molecule. The designed bursal virus B cell and T cell epitopes and interleukin 15 molecular polypeptide were co-expressed in Escherichia coli in series, and after fermentation, purification, emulsification and other processes, the bursal protein engineering vaccine with ideal immunogenicity was obtained. The vaccine prepared by the invention can effectively prevent pseudobursal disease.
[0024] Comprehensive analysis of domestic bursal virus epidemic strain genome sequence, antigen structure, epidemi...
Embodiment 2
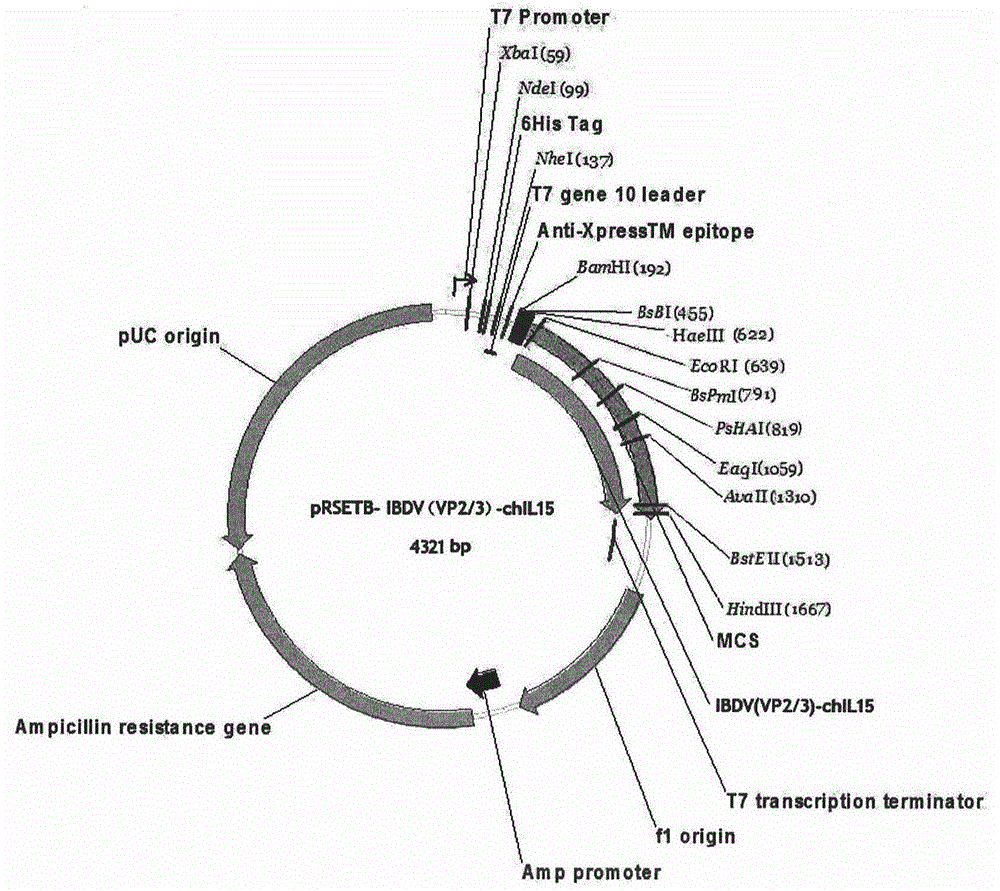
[0026] Example 2 Construction of Escherichia coli expression vector and expression strain
[0027] The designed polypeptide-encoding nucleotides were sent to Shanghai Handsome Biotechnology Co., Ltd. for synthesis. BamH I (5' end) and HindIII (3' end) restriction enzyme sites were designed at both ends of the nucleotide fragment. After synthesis, they were respectively cloned into the pMD18T vector, and sequence determination confirmed that the inserted gene fragment was consistent with the designed sequence (see the sequence list). The recombinant plasmids were named pMD18T-IBDV(VP2 / 3)-chIL15, respectively. The two plasmids were digested with the corresponding restriction enzymes. The E. coli expression vector was the pRSETB plasmid from Invitrogen Company, and the same restriction enzymes were also used for treatment. Digestion conditions: 10 μl reaction system, adding 2 μl of plasmid, 5 activity units of restriction endonuclease (New England Biolabs), 1 μl of 10× buffer wa...
Embodiment 3
[0031] Example 3 Fermentation, purification and emulsification of engineering bacteria
[0032] Fermentation Take the production strains, inoculate them in 2ml LB liquid medium (containing 100μg / ml ampicillin), and culture them at 37°C with shaking at 200rpm for 12 hours to activate the strains. Then inoculate the shake flask with an inoculation amount of 1:100, shake and culture at 37°C until OD600=3, and then inoculate it into a fermenter at a ratio of 10%. The medium used for fermentation is a semi-synthetic medium prepared with distilled water and does not contain any antibiotics. Calibrate the dissolved oxygen and pH electrodes, start the tank to stir, the rotation speed is 300rpm, and sterilize the tank on-line. When the temperature of the culture solution in the tank drops to 37.0°C, calibrate the pH and dissolved oxygen (OD) zero point. The fermentation temperature is 37.0±0.1°C, the dissolved oxygen is controlled at about 40%, and the pH is controlled at 7.0. When th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com