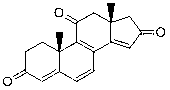
Androstane-4, 6, 8 (9), 13 (14)-tetraene-3, 11, 16-triketone and application thereof
A technology of androsteroids and triketones, applied in steroids, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
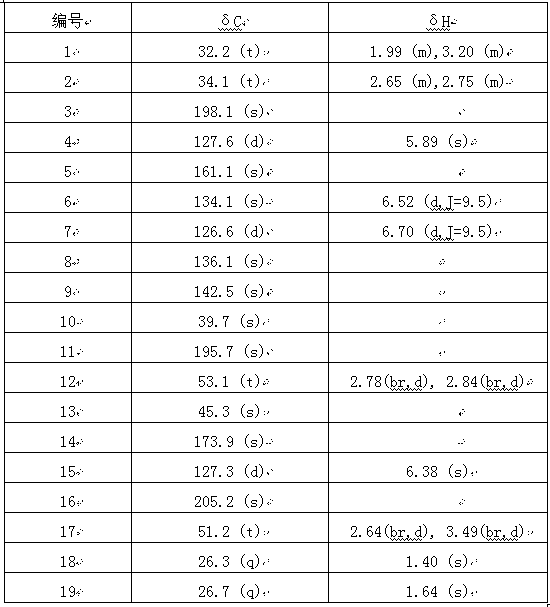
[0011] Example 1: Preparation of the compound androst-4,6,8(9),13(14)-tetraen-3,11,16-trione
[0012] Take Simao Vine ( Epigynum auritum ) Whole plant dry powder 8.3kg, extracted with ethanol 3 times, 24 hours each time, filtered to remove slag, concentrated and recovered ethanol to obtain an extract, then suspended the extract in water, first extracted with petroleum ether to remove low polar components , and then extracted with ethyl acetate, collected 150 g of the ethyl acetate extract, crudely separated with macroporous adsorption resin D101, and eluted with ethanol-water solution with a mass percentage concentration of 20%, 35% and 60% respectively, and collected 60% ethanol-water eluent was concentrated to obtain 20 grams of extract, mixed with 200-300 mesh silica gel, dry-packed for column chromatography, and used petroleum ether-acetone (volume ratio 10:1-1: 1) The two-phase system is the mobile phase for gradient elution, detected by thin-layer chromatography (TLC), ...
Embodiment 2
[0021] Example 2: Androst-4,6,8(9),13(14)-tetraene-3,11,16-trione immunosuppressive activity test
[0022] (1) Preparation of splenocyte suspension
[0023] Healthy Balb / c mice were killed by eyeball bleeding, soaked in 75% alcohol and disinfected for 5 minutes, taken out, placed in a sterile tray with the left side up, and in the ultra-clean bench, use sterilized tweezers to pick up the fur in the middle of the abdomen , make an incision, use another set of instruments to cut open the layers of the abdominal wall, use the third set of instruments to take out the spleen, remove fat and connective tissue, put it in PBS (phosphate buffer saline), wash away the floating blood; then the spleen Move the tissue to a plate containing RPMI 1640 incomplete culture medium, cut it into small pieces with scissors, grind the spleen in a 200-mesh stainless steel screen with a sterile syringe core, wash it with a small amount of PBS several times, and filter to obtain a single cell Su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com