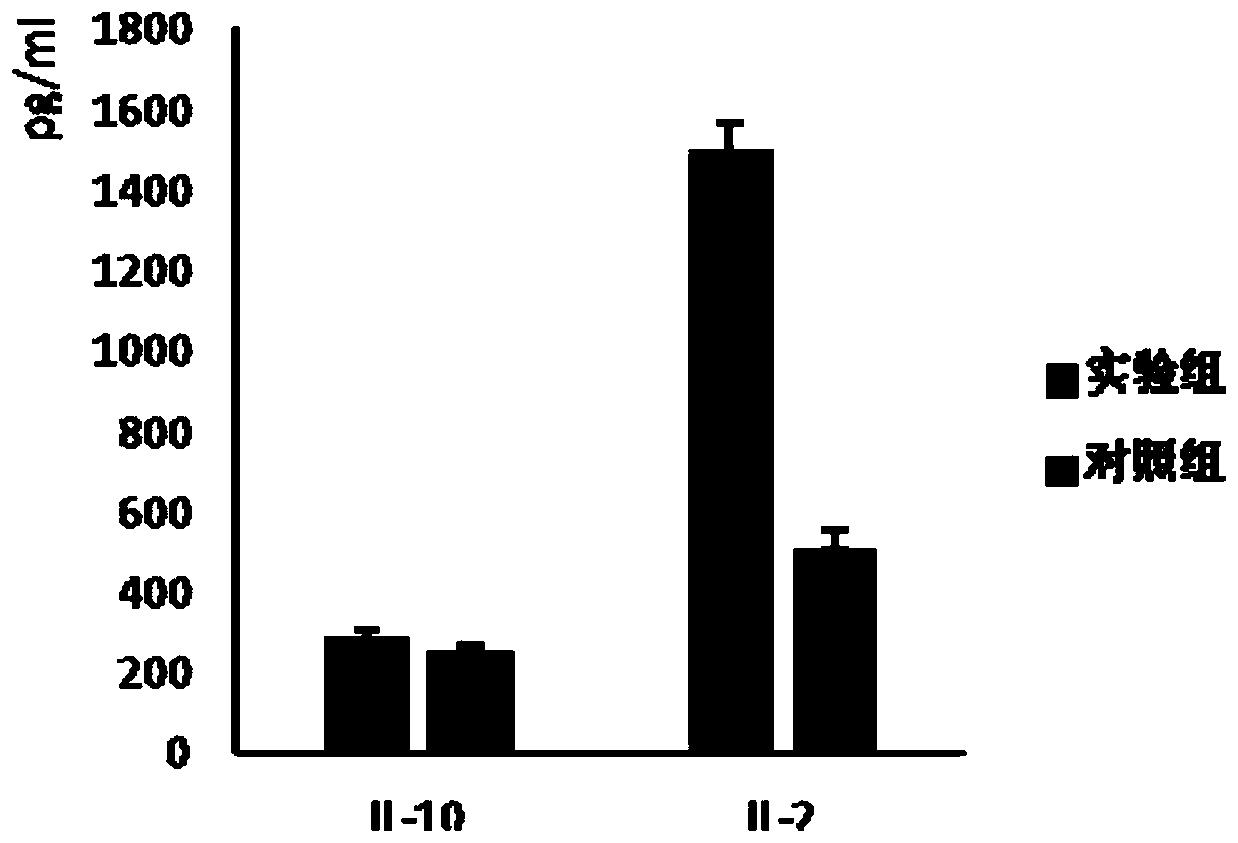
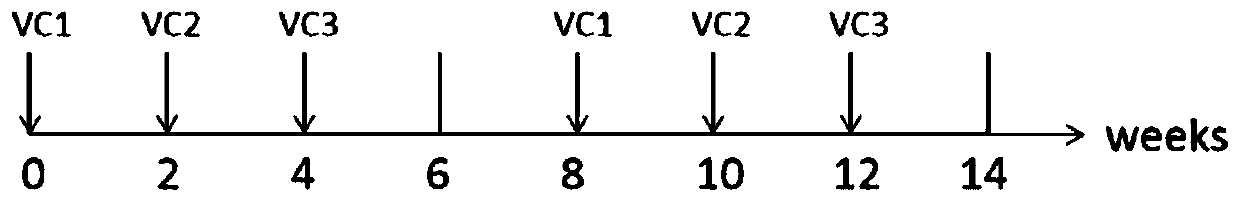
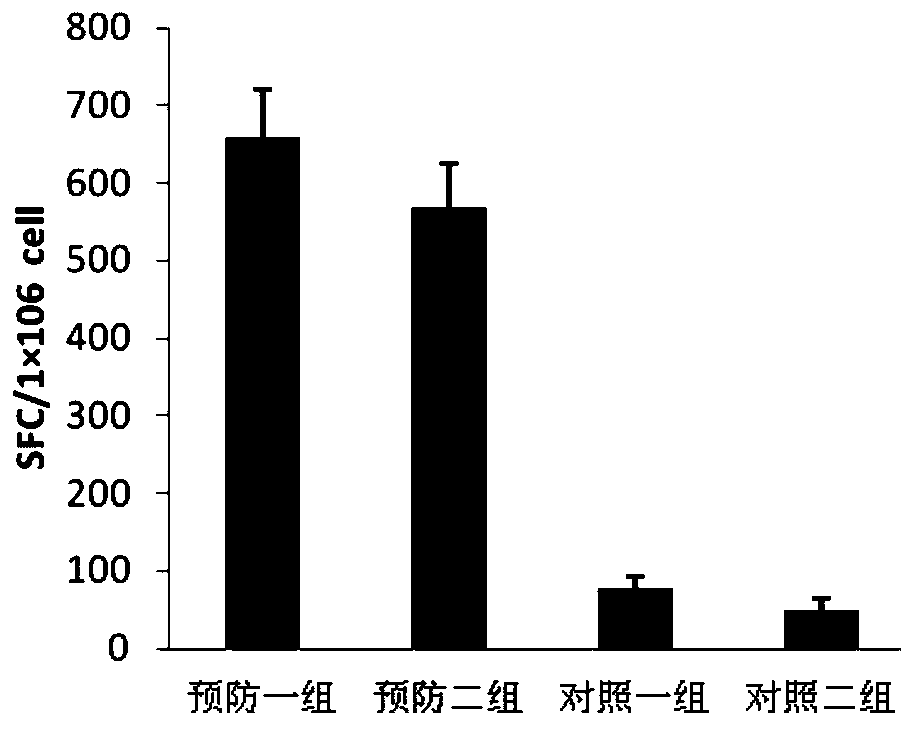
Therapeutic DC composite vaccine against herpes simplex viruses and preparation method thereof
A herpes simplex virus, therapeutic technology, applied in the field of vaccines, to increase transcription and expression, reduce lesions, and enhance immune effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The antigen gene fragments GM-CSF-gC+US10, GM-CSF-gD+ICP4, and GM-CSF-UL47 were respectively inserted into the lentiviral expression vector pLent-C-GFP, and three corresponding plasmids were prepared, and the gC+US10 Nucleic acid artificial sequence, gD+ICP4 nucleic acid artificial sequence, and UL47 nucleic acid artificial sequence are respectively added with NotI and AsiSI site restriction sites at both ends to synthesize a complete expression frame, which is inserted into the lentiviral pLent-C-GFP vector after restriction digestion (Invitrogen) NotI-AsiSI site, transformed into E.coli (Top10), after the correct identification by nucleic acid sequencing, use the endotoxin-free plasmid purification kit to extract and purify the plasmid to obtain the recombinant expression vector plasmid
[0042] In more detail, this embodiment adopts the following steps:
[0043] The antigen gene fragments GM-CSF-gC+US10, GM-CSF-gD+ICP4, and GM-CSF-UL47 were respectively inserted into...
Embodiment 2
[0047] Inoculate the lentiviral packaging cell line 293T in a 10cm culture dish containing DMEM+10% FBS; prepare the reaction system, mix well, and place it at room temperature for 20 minutes, then evenly add it dropwise to the culture dish containing 293T cells, and then place it in a CO2 incubator Medium culture; culture after transfection, to obtain lentivirus virus liquid carrying GM-CSF-gC+US10, GM-CSF-gD+ICP4, GM-CSF-UL47 encoding genes.
[0048] In more detail, this embodiment adopts the following specific steps:
[0049] Use the Lentiviral Packaging Kit lentiviral packaging kit, the specific method is as follows: inoculate the lentiviral packaging cell line 293T in a 10cm culture dish containing DMEM+10%FBS, culture at 37°C, 5% CO2, and the adhesion rate is 70% -80% ready for transfection. Take a sterile 1.5ml EP tube or 15ml centrifuge tube, and prepare the reaction system according to the following components: serum-free DMEM: 4ml; pLent-gC+US10, pLent-gC+US10, pLen...
Embodiment 3
[0051] Collect peripheral venous blood from patients with herpes simplex virus, isolate peripheral blood mononuclear cells, retain adherent cells, add cytokines to induce mononuclear cells to differentiate into DC, and culture imDC.
[0052] In more detail, this embodiment adopts the following specific steps:
[0053] Collect 50 ml of peripheral venous blood from patients with herpes simplex virus, use TBD sample density separation medium (purchased from Tianjin Haoyang Huake Biology), separate peripheral blood mononuclear cells, and culture them in 10 ml of culture medium (purchased from TaKaRa Company, GT-T551), After culturing at 37°C and 5% CO2 for 3-4 hours, discard the suspended T cells, keep the adherent cells, and add cytokines rhIL-4 (final concentration: 50ng / ml), rhGM-GSF (final concentration 100ng / ml) to induce mononuclear cells to differentiate into DCs, the medium was replaced every 48 hours, and imDCs were obtained on the 5th day of culture.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com