Recombinant adenovirus vaccine for African swine fever and construction method of recombinant adenovirus vaccine
A technology of African swine fever virus and recombinant adenovirus, applied in the field of genetic engineering technology and immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1 Construction of Adenoviral Vector Plasmid pAd5 Deleting E1 and E3 Genes
[0136] in A549 cells Amplified wild-type human adenovirus type 5 ( VR-1516, gene sequence AC_000008.1) virus, collect and concentrate the virus liquid, use the HirtVirual DNA Extract method to extract the adenovirus genome, use the cosmid method to construct the linear hAd5 genome into a circular supercos-Ad5 vector plasmid, use CRISPR / cas9 excises the E1 region of hAd5 adenovirus, and the designed gRNA is as follows:
[0137] hAd5-E1 upstream gRNA:
[0138] GGCGGGAAAACUGAAUAAGGUUUUAGAGCUAGAAAUAGCAAGUUAAAAUAAGGCUAGUCCGUUAUCAACUUGAAAAAGUGGCACCGAGUCGGUGCUUUU
[0139] hAd5-E1 downstream gRNA:
[0140] GAGAUGAUCCAGUCGUAGCGUUUUAGAGCUAGAAAUAGCAAGUUAAAAUAAGGCUAGUCCGUUAUCAACUUGAAAAAGUGGCACCGAGUCGGUGCUUUU
[0141] Design gRNA sites in the upstream and downstream of the hAd5 E1 region, recover the large fragment vector after cutting, design primers, insert the ITR and PIX sequences into the...
Embodiment 2
[0147] Example 2 Construction of Adenoviral Vector Plasmid pAd5ΔE4 Deleting E1, E3 and E4 Genes
[0148] Using the carrier plasmid pAd5 obtained in Example 1 that has knocked out the E1 and E3 genes, further knocking out the E4 gene can increase the capacity of the adenovirus vector and reduce its immunogenicity, and use PCR to amplify part of the fiber and Introduce the NdeI single restriction site, and then use Gibson's seamless cloning method to connect the redundant excised fragments to the vector to obtain a vector plasmid that deletes the E1, E3 and E4 genes and introduces the SwaI and I-sceI restriction sites pAd5ΔE4.
[0149] 1. Selection of target gene E4 CRISPR target sequence
[0150] 1) Selection of CRISPR target sequence of fiber gene upstream of E4 gene
[0151] Using Thermo Fisher GeneArt TM The CRISPR Search and Design tool (thermofisher.com / crisprdesign) software inputs the first 400 bases of the fiber gene, and the software automatically analyzes the seque...
Embodiment 3
[0228] Example 3 Construction of Adenoviral Vector Plasmid pAd5LCL3 Deleting E1, E3, E4 and E2a Genes
[0229] 1. Selection of target gene E2a CRISPR target sequence
[0230] 1) Selection of CRISPR target sequence of 100k genes upstream of E2a gene
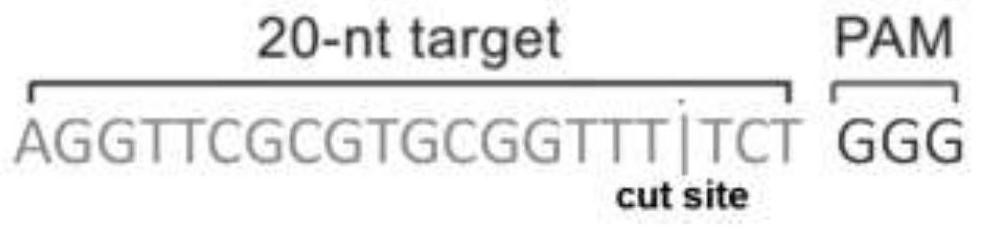
[0231] Using Thermo Fisher GeneArt TM CRISPR Search and Design tool (thermofisher.com / crisprdesign) software, input the first 400 bases of 100k genes, the software automatically analyzes the sequence of 400 bases, and provides 6 potential CRISPR target sequences. Considering the length of the E2a gene knockout sequence and the requirements for constructing live vectors, ATAGGTGGCGTTCGTAGGCA was selected as the targeting sequence, and the finally obtained gRNA was named 100k-gRNA, and the cleavage site and PAM site were as follows: Figure 8 shown.
[0232] 2) Selection of CRISPR target sequences in the downstream non-coding sequence of E2a
[0233] Using Thermo Fisher GeneArt TM CRISPR Search and Design tool (thermofisher.com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com