Oral vaccine delivery system and application thereof
A drug delivery system and oral vaccine technology, applied in the field of new pharmaceutical preparations, can solve the problems of small size of nanoparticles, loss of nanoparticles, large surface activity, etc., and achieve good biocompatibility, simple process, and improved immunogenicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Scheme screening of acid-resistant HP55 / PLGA-CCF NPs
[0060] Option One:
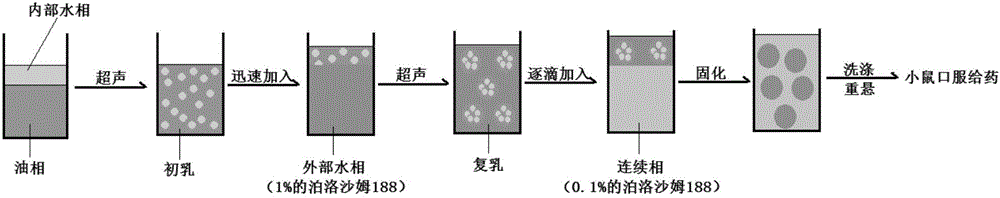
[0061] (1) Weigh 40mg of PLGA and 20mg of HP55 in the prescribed amount, dissolve in a mixed solution of 1mL of acetone and 1mL of dichloromethane, and prepare the drug-loaded organic phase for use;
[0062] (2) Weigh 60 mg of poloxamer 188 and dissolve in 6 mL of ultrapure water to make a hydration medium solution as W 1 / O / W 2 The external aqueous phase of the system. Weigh 30mg of poloxamer 188 and dissolve it in 30mL of ultrapure water to make a hydration medium solution, which is used as the continuous phase for nanoparticle dispersion;
[0063] (3) Dissolve the dual epitope-dual adjuvant Helicobacter pylori vaccine CTB-UE-CF (CCF) in refolding buffer (50mmol / L NaH 2 PO4, 10 mmol / L TisHCl, 2 mmol / L GSH, 0.4 mmol / L GSSH, adjust the pH value to 8.0 with HCl or NaOH), the concentration is about 4.2mg / mL, as the internal water phase of the double emulsion (W 1 ). Add 500 μL of ...
Embodiment 2
[0073] Example 2: Dynamic research on immune level of HP55 / PLGA-CCF NPs vaccine.
[0074] (1) Grouping scheme of SPF BALB / c mice: (A) NC group: Mice were orally administered a suspension of 500 μL PBS and aluminum hydroxide adjuvant. (B) HP55 / PLGA-group: Mice were orally administered acid-resistant HP55 / PLGA nanoparticles without antigen encapsulation. (C) HP55 / PLGA-CCF group: Mice were orally administered acid-resistant HP55 / PLGA nanoparticles encapsulated with 100 μg CCF. (D) PLGA-CCF group: Mice were orally administered ordinary PLGA nanoparticles encapsulated with 100 μg CCF. (E) Alum-CCF group: Mice were orally administered a suspension of 100 μg CCF and aluminum hydroxide adjuvant.
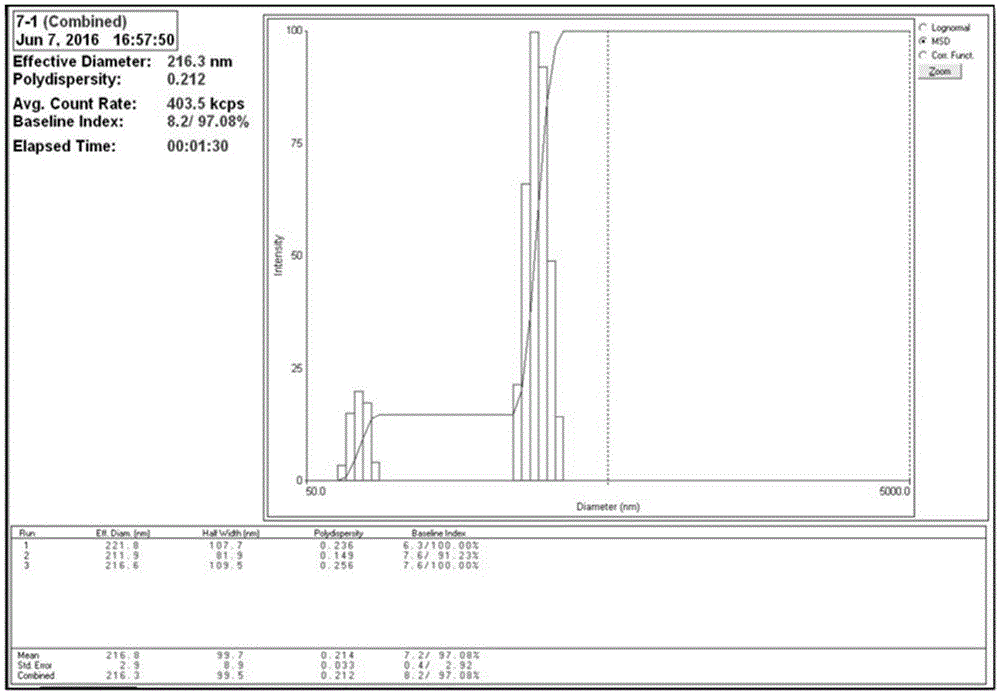
[0075] (2) Mice immunization and challenge program: according to Image 6 , orally immunized 4 times by intragastric administration, with an interval of 7 days each time, and challenged the immunized mice two weeks later: intragastric administration of Hp suspension (1×10 9 CFU / mL), 0.3...
Embodiment 3
[0081] Example 3: HP55 / PLGA-CCF NPs vaccine induces a high level of systemic immune response.
[0082] ELISA detection of specific antibodies IgG, IgM and IgA in antiserum before and after challenge:
[0083] ELISA reagents: (A) Coating solution: Beijing Solaibao Technology Co., Ltd. (B) Washing solution: weigh 0.2g KH 2 PO 4 , 2.9gNa 2 HPO 4 •12H 2 O, 8.0g NaCl, 0.2g KCl, 0.5mL Tween-20, add ddH 2 O was adjusted to 1000mL (PBST). (C) Blocking solution: Weigh 3.0 g of BSA and dissolve in 100 mL of washing buffer, filter and sterilize and store at 4°C. (D) Sample diluent: Weigh 1.0 g of BSA and dissolve in 100 mL of washing buffer, filter and sterilize and store at 4°C. (E) Substrate solution: soluble one-component TMB substrate solution. (F) Stop solution: measure 178.3mL of distilled water, add 21.7mL of concentrated sulfuric acid (1M H 2 SO 4 ).
[0084] Detection protocol: Dilute the natural urease antigen to 10 μg / mL with coating solution, add 100 μL to each we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com