HLA-A11 restricted GPC3-derived polypeptide and vaccine containing polypeptide
A technology of HLA-A11 and peptide vaccines, applied in the field of biopharmaceuticals, to achieve the effect of improving immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 blood collection
[0048] The inventor of this application signed an informed consent form with the HLA-A11 liver cancer patient, and the patient was treated in the Tumor Interventional Therapy Center of Beijing You'an Hospital Affiliated to Capital Medical University. 10 ml of venous blood was drawn from the patient, and peripheral blood mononuclear cells were routinely isolated by Ficoll density gradient centrifugation according to a previously reported method.
Embodiment 2E
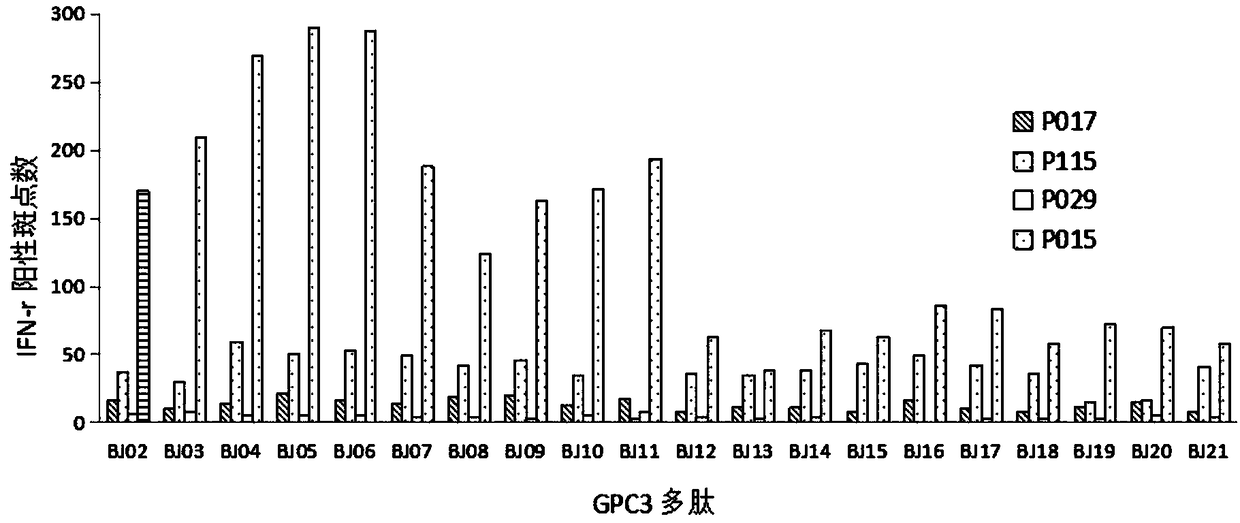
[0049] Example 2 ELISPOT method analysis of GPC3 specific killer T cell activity
[0050] The inventors of the present application isolated peripheral blood mononuclear cells from patients with HLA-A11 positive hepatocellular carcinoma and incubated them with GPC3 polypeptides, namely BJ4:443-451, BJ5:289-297, and BJ6:472-480, to detect whether there is GPC3 antigen specificity effector T cells.
[0051] The ELISPOT method was used to detect whether there were killer T cells that could specifically react with GPC3 and produce IFN-γ. Human IFN-γ ELISPOT kit (BD product) was used to detect whether IFN-γ was produced. If effector cells respond to GPC3-presenting cells and secrete IFN-γ, each IFN-γ molecule produces a brownish-red spot in the assay system.
[0052] First, the ELISPOT plate (BD) was coated with anti-human IFN-γ antibody for 18 hours, and blocked with 10% FCS RPMI1640 for 2 hours. Peripheral blood mononuclear cells (200 μl) were mixed with GPC3 peptide (10 μg) an...
Embodiment 3
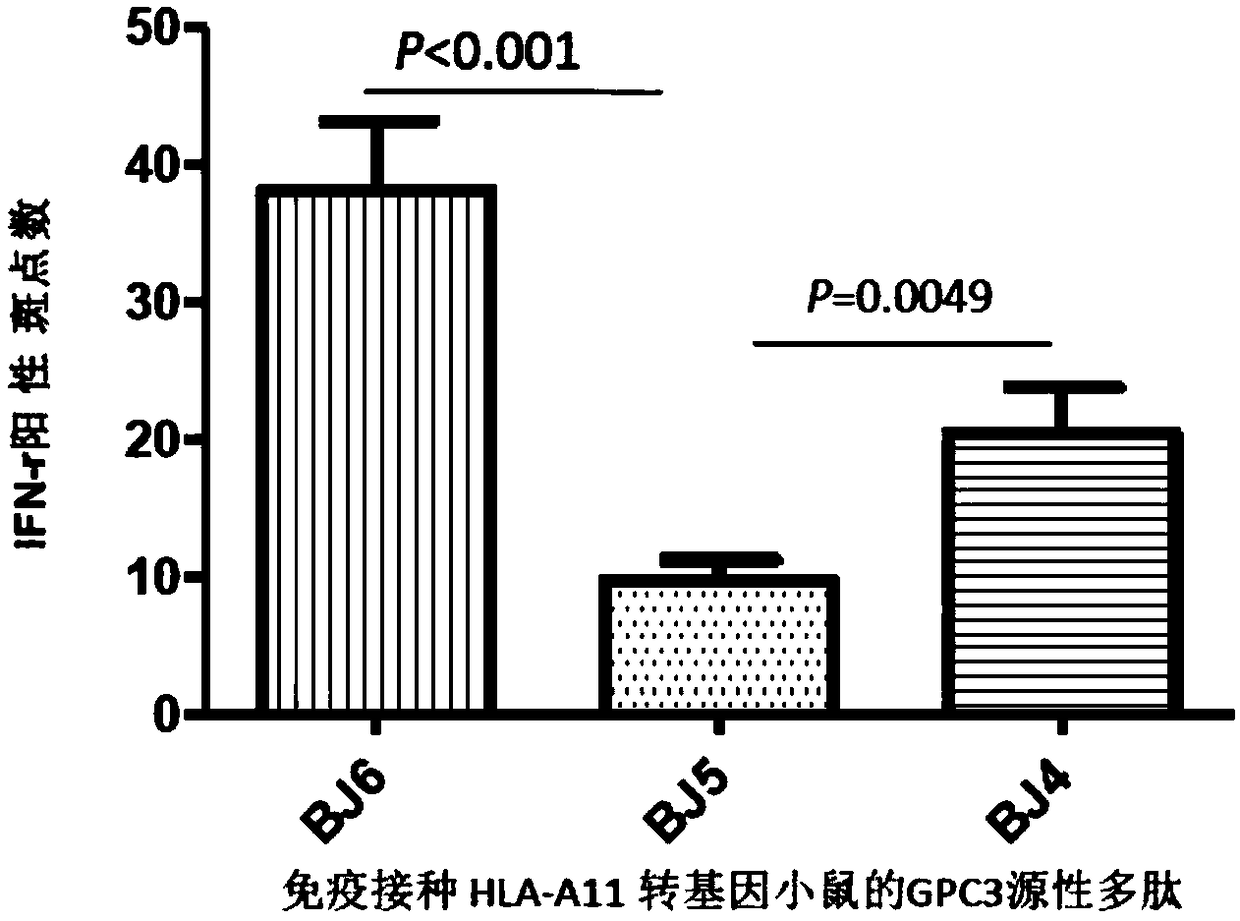
[0053] Example 3 Detection of polypeptide immunogenicity
[0054] For immunization, polypeptide vaccines must have good immunogenicity and immunoreactivity, otherwise they cannot induce specific immune responses for immunoprophylaxis and immunotherapy. To this end, the inventors of the present application identified the polypeptide site, detected the immunoreactivity between the polypeptide and T cells of liver cancer patients, then inoculated the polypeptide into transgenic mice to detect the immunogenicity, and developed the HLA-A11-restricted GPC3-derived polypeptide vaccine in three steps .
[0055] In order to determine whether the polypeptide can induce GPC3 polypeptide-specific effector T cell responses when immunized as a vaccine, the inventors of the present application used human HLA-A11 transgenic mice to immunize with GPC3 polypeptide, and then immunized the polypeptide with the spleen of the immunized mice. The cells were co-cultured, and ELISPOT was used to dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com