Meningococcus vaccine
A meningococcal and vaccine technology, which is applied in the direction of antibacterial drugs, medical preparations with non-active ingredients, carrier-bound antigen/hapten components, etc., can solve problems that do not involve joint effects, and achieve enhanced specific immune responses, The effect of reducing the cost of vaccines and improving the effect of immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. CpG-ODN can enhance the immune response of mice to the chemical conjugate of group A meningococcal capsular polysaccharide and tetanus toxoid
[0038] The chemistry of preparing group A meningococcal capsular polysaccharide and group A meningococcal capsular polysaccharide and tetanus toxoid according to the method described in patent document CN101007167A (see Example 1 and Example 4 on pages 9-12 of the specification) Conjugate (hereinafter referred to as "Ag"). In the chemical conjugate, the polysaccharide content is 106 μg / ml, and the carrier protein content is 269 μg / ml.
[0039] The CpG-ODN used in this example was designed by the inventors, and the sequence is as follows: 5'-TCGTTCGTTCGTTCGTTCGTT-3', artificially synthesized by Shanghai Shenggong Biotechnology Company, and subjected to full chain thio modification, polyacrylamide gel electrophoresis (PAGE) Purified, dissolved in physiological saline, stored in a refrigerator at -20℃ for later use.
[0040] ...
Embodiment 2
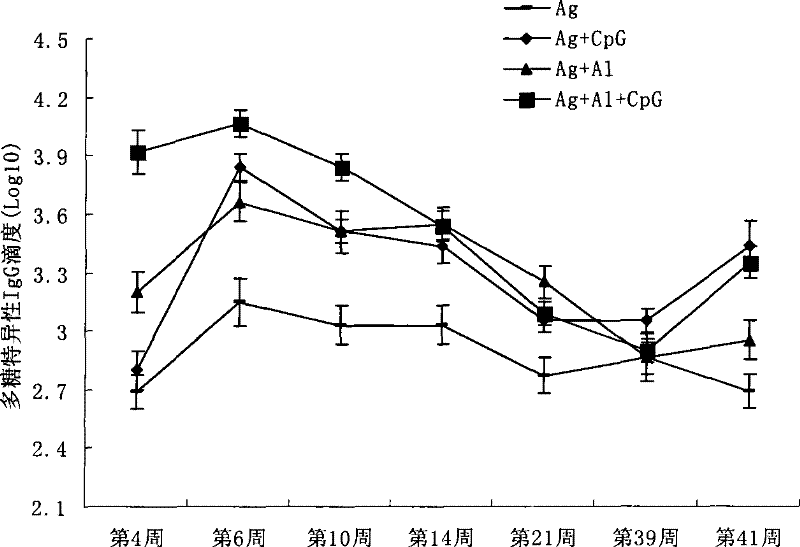
[0047] Example 2. CpG-ODN can enhance the persistence of mice immune response to the chemical conjugate of group A meningococcal capsular polysaccharide and tetanus toxoid
[0048] The Balb / c mice were immunized according to the same method as in Example 1, and then the meningococcal capsular polysaccharide specific IgG antibody titers were detected to evaluate the effect of CpG-ODN on group A meningococcal capsular polysaccharide in mice The difference between the chemical conjugate and the tetanus toxoid chemical conjugate on the durability of the immune response is that the same method is used to boost the immunization in the 4th week and 39th week after the initial immunization, and the 4th and 6th week after the initial immunization The blood was collected at week, week 10, week 14, week 21, week 39, and week 41, respectively, and the serum was separated, and the meningococcal polysaccharide-specific IgG total antibody was detected according to the method described in Example...
Embodiment 3
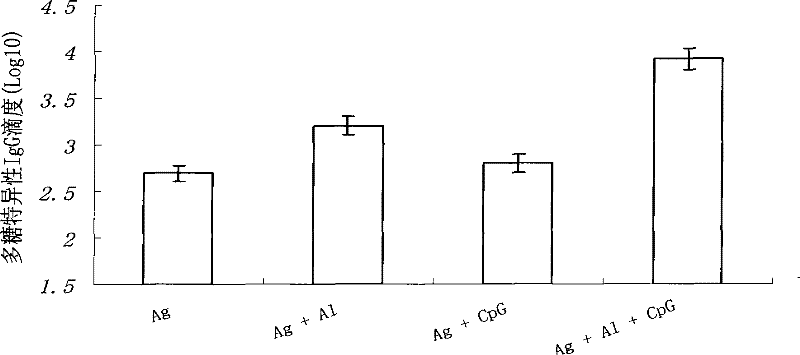
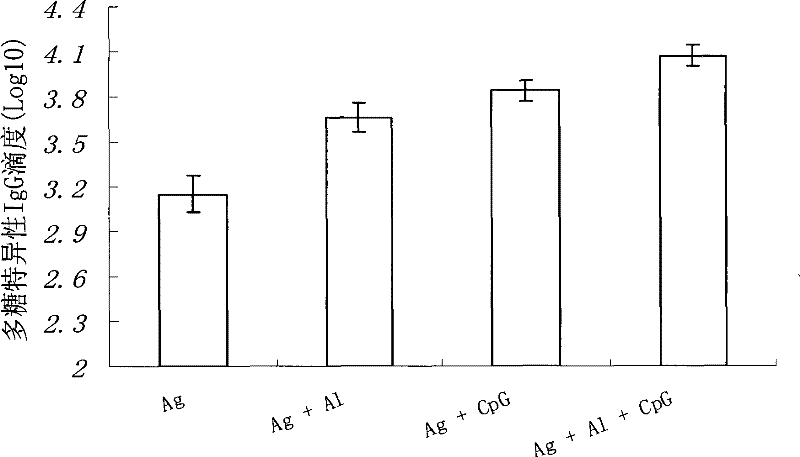
[0050] Example 3. CpG-ODN can reduce the amount of chemical conjugate of group A meningococcal capsular polysaccharide and tetanus toxoid
[0051] The Balb / c mice were immunized according to the same method as in Example 1, and then the meningococcal capsular polysaccharide specific IgG antibody titer was detected to determine the effect of CpG-ODN on the group A meningococcal capsular polysaccharide and tetanus The difference in the amount of chemical conjugate of toxin is that the amount of meningococcal capsular polysaccharide and the chemical conjugate of tetanus toxoid are different, and the dosage is the original double dose (2.2μg Ag / mouse ), quarter dose (0.55μg Ag / mouse) and one sixteenth dose (0.14μg Ag / mouse). See the result Figure 3a with Figure 3b .
[0052] Figure 3a with Figure 3b The results shown are the polysaccharide-specific IgG antibody titers at the 4th week after the initial immunization and the second week after the booster immunization. It can be seen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com