Recombinant pasteurella multocida toxin protein and application thereof
A pasteurella, recombinant protein technology, applied in antibacterial immunoglobulin, application, complement protein and other directions, can solve the problems of reduced feed utilization efficiency, pig jaundice, high pollution rate, etc., to improve immunity and efficiency, The effect of increasing the specific immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 Construction of Pasteurella septica toxin recombinant protein (rPMT)
[0060] 1. Design of the amino acid sequence of Pasteurella septica toxin recombinant protein 1 (rPMT1)
[0061] First, using the full-length amino acid sequence of Pasteurella septica toxin protein (PMT) (as shown in SEQ ID NO: 1) as a template, using an epitope prediction website (such as, but not limited to, BCPred( http: / / ailab.cs.iastate.edu / bcpreds / )) Predicted epitopes of Pasteurella septica toxin protein (PMT).
[0062] The epitopes predicted by Epi are shown in Table 1 (SEQ ID NOs:20–42). These epitopes are divided into three segments, numbered Epi1, Epi2 and Epi3 respectively, and their amino acid sequences are shown in SEQ ID NO: 2, 3, and 4; Epi1, Epi2, and Epi3 contain each epitope shown in Table 1, respectively.
[0063] Table 1 Epitope prediction results of Pasteurella septica toxin protein (PMT)
[0064] Divide into sections
sequence
SEQ ID NO.
...
Embodiment 2
[0069] Embodiment 2 The cultivation of Bordetella bronchiseptica and Pasteurella multocida
[0070] 1. Culture of Bordetella bronchiseptica (B.bronchiseptica)
[0071] In this example, Bordetella bronchiseptica (B. bronchiseptica) was distributed from the Animal Health Laboratory of the Council of Agriculture, Executive Yuan (Taiwan).
[0072]First inoculate the Bordetella bronchiseptica (B. bronchiseptica) on TSB solid medium [containing 5% (v / v) yeast extract, 10% (v / v) serum, soybean decomposition Protein casein medium (tryptic soy broth, TSB, BD Company, USA)], after culturing overnight at 37°C, a single colony was selected and inoculated in brain heart infusion (BHI) liquid medium (BD Company, USA) ), shake culture overnight at 37°C; then take the bacterial solution and inoculate it in BHI liquid medium, culture shake overnight at 37°C, and calculate the colony forming unit (CFU) value; finally add formaldehyde (formaldehyde ), shaking at room temperature for 24 to 36 h...
Embodiment 3
[0076] Example 3 Preparation of the porcine atrophic rhinitis immune composition containing Pasteurella septica toxin recombinant protein (rPMT)
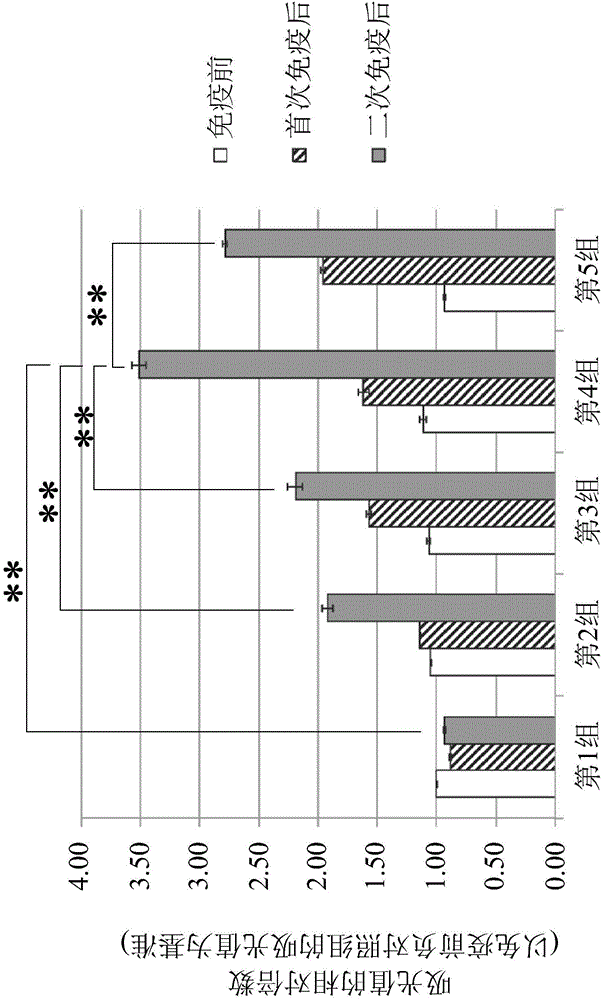
[0077] The Pasteurella septica toxin recombinant protein (rPMT) (SEQ ID NO:10 or 11) (final concentration is 65 μ g / ml) obtained in embodiment one and the inactivated bronchial septic Bordet obtained in embodiment two B. bronchiseptica (final concentration 1x10 9 CFU / ml), inactivated Pasteurella septica type A (PmA) (final concentration 1x10 9 CFU / ml) and inactivated Pasteurella septica type D bacteria (PmD) (final concentration is 1x10 9 CFU / ml) was mixed evenly with phosphate buffered solution (PBS), and aluminum gel [final concentration was 30% (v / v)] was added as an adjuvant to prepare a porcine atrophic rhinitis immune composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com