Tuberculosis subunit vaccine containing unite adjuvant
A subunit vaccine and combined adjuvant technology, applied in the field of new subunit vaccines, can solve the problems of weak immunogenicity and poor response effect, and achieve the effects of enhancing cellular immunity, enhancing Th1 type response, and enhancing humoral immune response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1Ag85
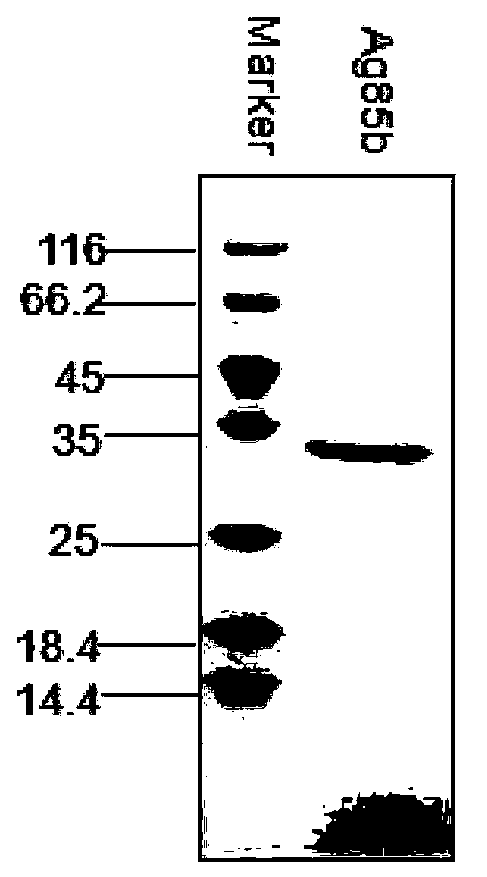
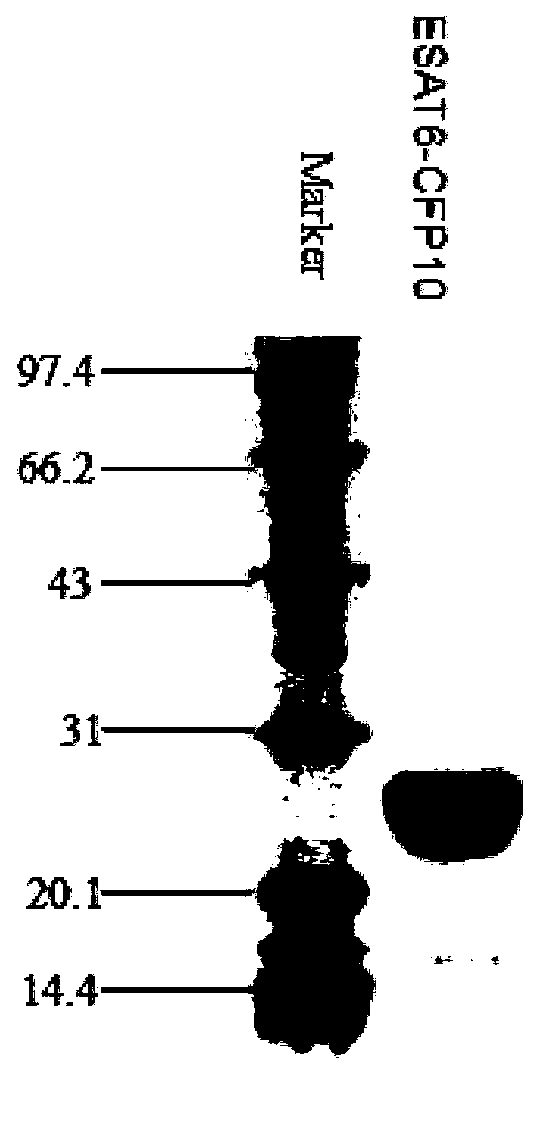
[0024] Example 1 Preparation of Ag85b and ESAT6-CFP10 antigens
[0025] 1.1 Test method
[0026] 1) Amplification of Ag85b gene and purification of protein
[0027] Query the nucleic acid and amino acid sequences of Mycobacterium tuberculosis H37Rv antigen ESAT6 and CFP10 from Genbank, and design primers according to the target sequence.
[0028] Table 1
[0029]
[0030] First, the H37Rv whole genome was used as a template, and the primers are shown in Table 1. Ag85b was amplified in a 50μL PCR system (ddH 2 O30.5μL, 10×Buffer 5μL, Taq enzyme 0.5μL, dNTP4μL, upstream primer 4μL, downstream primer 4μL, DNA template 2μL). The amplification conditions were: 94°C for 10 min; 94°C for 45s, 62.2°C for 45s, 72°C for 1 min, 33 cycles; 72°C for 5 min.
[0031] After agarose gel electrophoresis of the PCR product, the gel was recovered to obtain the target fragment, which was digested by NdeI and EcoRI and ligated with the pET30a cloning expression vector under the catalysis of T4 DNA ligase at ...
Embodiment 2
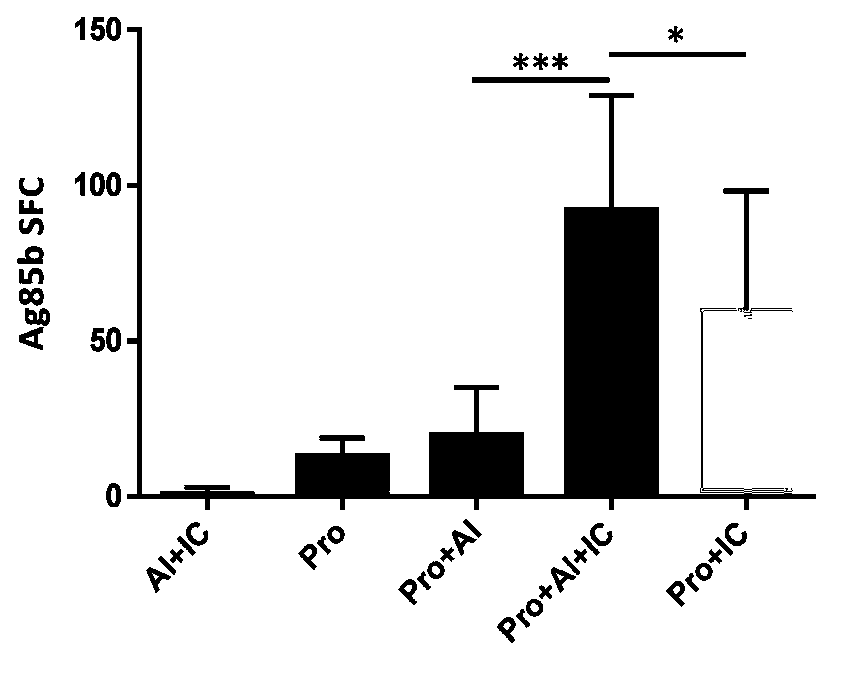
[0045] Example 2: Immunological study of the tuberculosis subunit vaccine containing the combined adjuvant in the present invention
[0046] 1. Material
[0047] Research objects: 30 SPF female BALB / c mice (6-8 weeks old)
[0048] 2. Methods and results
[0049] 2.1 Experimental design
[0050] Thirty female BALB / c mice were randomly divided into 5 groups, each with 6 mice. The mice were similar in age and body weight. The test groups are shown in Table 2: (EC is ESAT6-CFP10)
[0051] table 3
[0052] Group
Immunization dose ( / only)
Aluminum+Poly IC
Al(OH) 3 (0.2mg)+poly IC(50μg)
Ag85b(10μg)+EC(10μg)
Protein + Aluminum
Ag85b(10μg)+EC(10μg)+Al(OH) 3 (0.2mg)
[0053] Protein+PolyIC
Ag85b(10μg)+EC(10μg)+poly IC(50μg)
Protein + Aluminum + PolyIC
Ag85b(10μg)+EC(10μg)+Al(OH) 3 (0.2mg)+poly IC(50μg)
[0054] The mice were immunized with the above reagents intramuscularly on the hind legs of the mice, and immunized with 3 injections at 10 days intervals. Splenic...
Embodiment 3
[0058] Example 3 Animal protection test
[0059] 1. Experimental method
[0060] Immunotherapy of Mtb infection in guinea pigs
[0061] 16 SPF Hartley guinea pigs (300-350g / mouse), half male and half female, were divided into two groups (experimental group and control group), each with 8 animals. The experimental group and the control group guinea pigs were challenged subcutaneously 5.0×10 3 After CFU Mtb, the experimental group was treated with the reference vaccine, a total of 6 injections, with an interval of 2 weeks between each injection, the dose of each injection was [Ag85b(10μg)+EC(10μg)+Al(OH) 3 (0.2mg)+poly IC(50μg)] / 0.5mL / only. Guinea pigs in the control group were injected with the same amount of saline as a control. One week after the last immunization, the guinea pigs were dissected. Analyze the comprehensive pathological index of liver, spleen and lungs and the bacterial load of spleen and lungs.
[0062] Organ disease index score
[0063] After Mtb-infected guinea pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com