Ginseng total saponin or monomer saponin RbI vaccine immunological adjuvant application
A technology of immune adjuvant and total ginseng saponins, applied in the field of preparation of vaccine immune adjuvants by total ginsenosides or monomeric saponins Rb1, which can solve problems such as stimulation, toxicity, and complex components of propolis, and achieve short and extended validity periods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
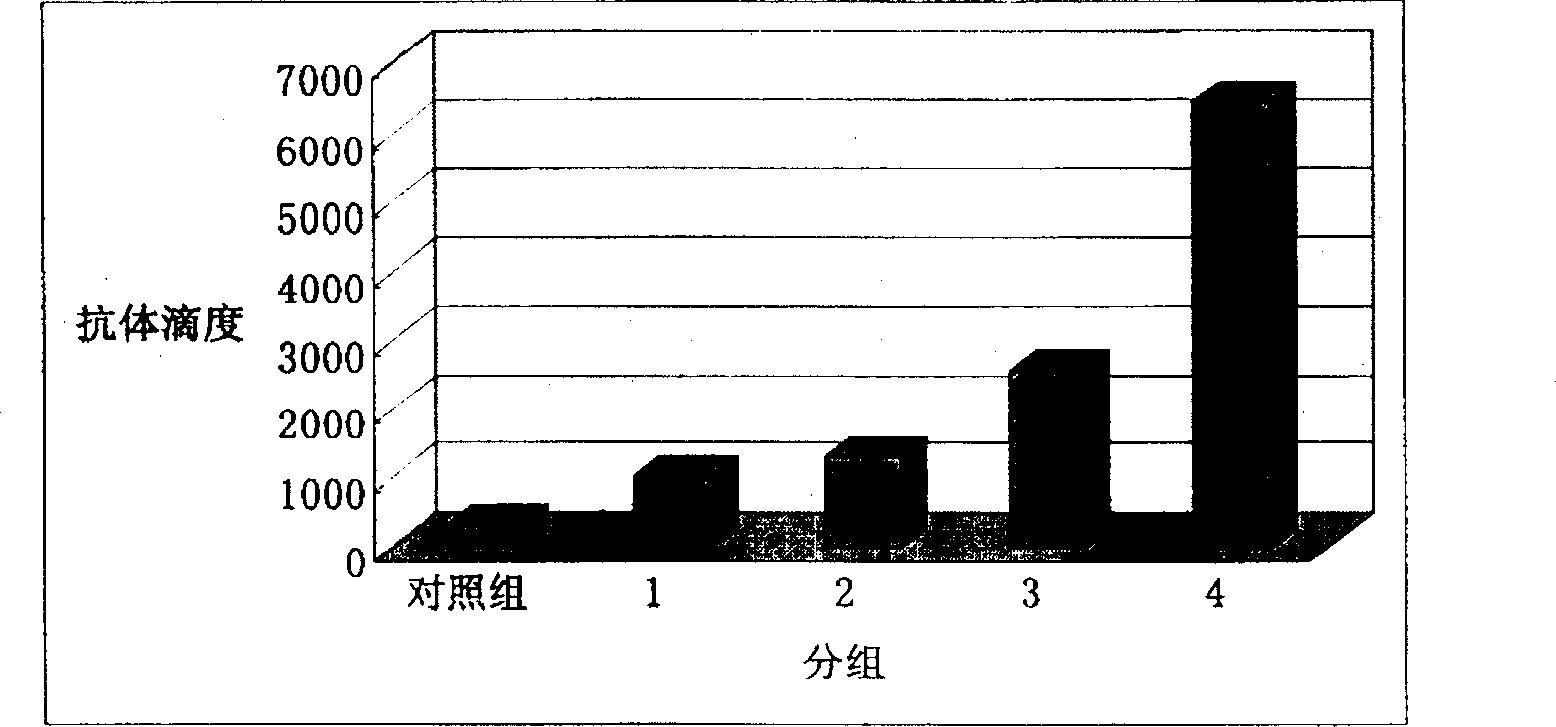
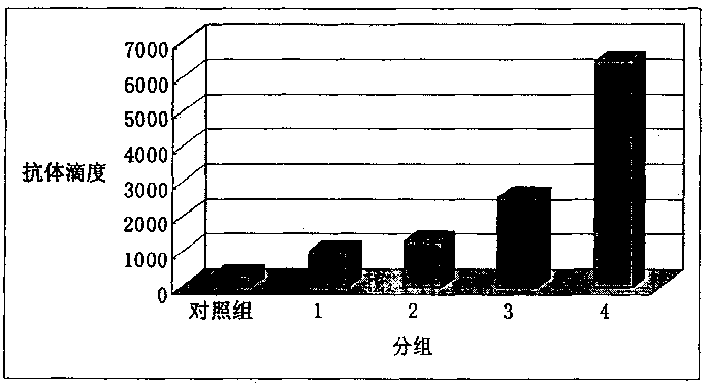
[0013] 1) Dissolving total ginsenosides or ginsenoside monomer Rb1 in physiological saline to prepare a solution of total ginsenosides and ginsenoside monomer Rb1. Mix inactivated Staphylococcus aureus and ginsenoside solution to prepare Staphylococcus aureus vaccines containing different adjuvants. Each milliliter of vaccine contains 10 6 Inactivated Staphylococcus aureus.
[0014] 2) Subcutaneously inject guinea pigs with 1 ml of the above vaccine. Two weeks later, the same method was used to inject once more. Before the first injection and 2 weeks after the second injection, blood samples were collected and serum was separated. Serum IgG content was detected by agar diffusion method. Elevated IgG levels indicate an enhanced humoral immune response.
[0015] Minute
Embodiment 2
[0017] 1) Dissolving total ginsenosides or ginsenoside monomer Rb1 in physiological saline at concentrations of 4 mg / ml and 1 mg / ml, respectively. Sterilize the solution through a 0.22 micron filter. Store at room temperature and set aside.
[0018] 2) After mixing 5 ml of commercial inactivated Staphylococcus aureus vaccine (containing aluminum hydroxide adjuvant) and 1 ml of total ginsenoside solution or 1 ml of ginsenoside monomer Rb1 solution, intramuscularly inject cows into the neck. Two weeks later, the same method was used to inject once more. After another 2 weeks, blood samples from cows were collected, blood lymphocytes were separated, and lymphocyte transformation tests were done. Calculate the leaching index. A high lymphatic index indicates a high cellular immune response.
[0019]
group
number of cows
Con A
PWM
golden yellow grapes
Coccal antigen
Inactivated Staphylococcus aureus vac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com