Purification method of candidate antigen pa5505 of Pseudomonas aeruginosa genetic engineering vaccine
A technology of PA5505, a genetically engineered vaccine, applied in the field of purification of the candidate antigen PA5505 of a genetically engineered vaccine of Pseudomonas aeruginosa, can solve the problems of no recombinant protein purification method, unknown structure and function, unclear physical and chemical properties, etc., and achieves good results. Immune protection, effect of high humoral immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Construction of recombinant engineering bacteria pGEX-6p-2-PA5505 / XL-1blue
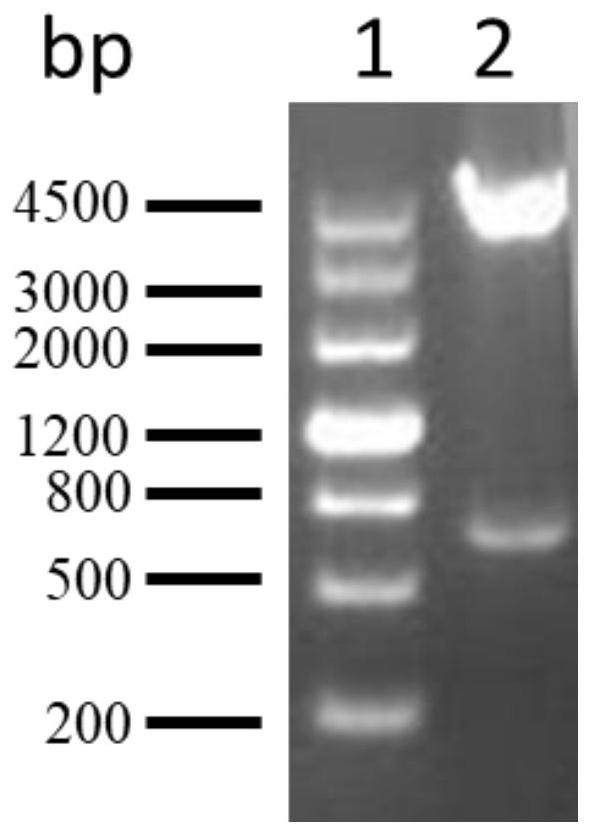
[0067] According to the coding sequence of PA5505, the upstream and downstream primers were designed, and the target sequence was obtained by PCR amplification. The target sequence and the pGEX-6p-2 vector were digested with BamH1 and Xho1 and ligated with T4 ligase to obtain the recombinant plasmid pGEX-6p- 2-PA5505( figure 2 It is the result of double enzyme digestion identification of the recombinant plasmid). The above-mentioned recombinant plasmid was transformed into competent Escherichia coli XL-1blue to obtain recombinant engineered bacteria pGEX-6p-2-PA5505 / XL-1 blue.
Embodiment 2
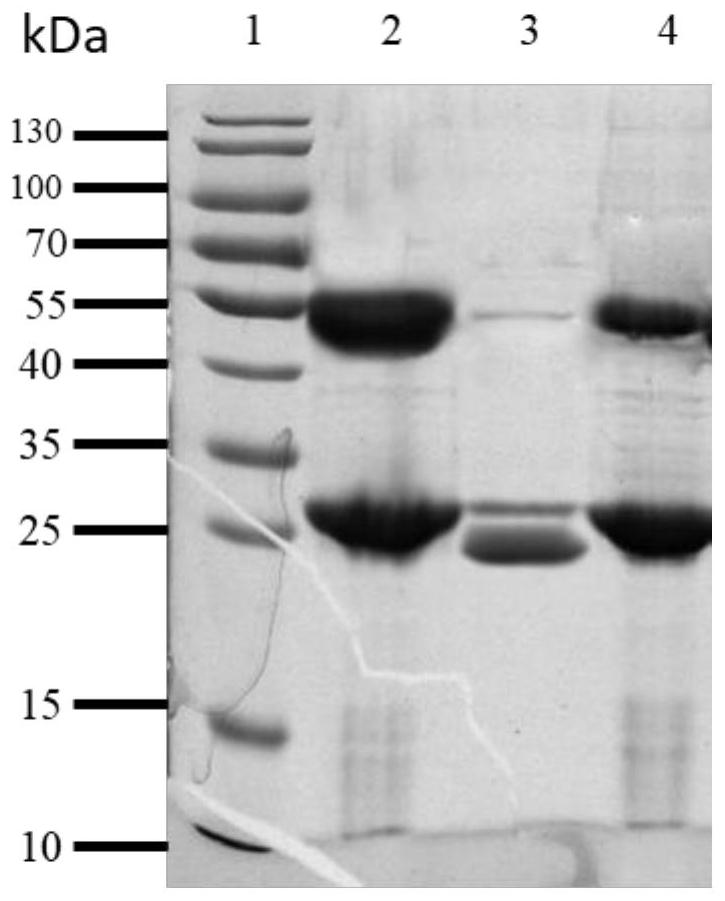
[0068] Example 2: Expression and enzyme digestion identification of PA5505
[0069] Take 200 μL of the pGEX-6p-2-PA5505 / XL-1 blue bacterial solution stored in the refrigerator at 4 °C and add it to 20 mL of LB medium containing Amp resistance for one activation. IPTG with a final concentration of 200 μM was placed in a shaker at 16°C for overnight induction. After the induction was over, the cells were collected by centrifugation at 5000 rpm for 10 minutes. After the cells were resuspended in 1.5 mL of PBS, the cells were ultrasonically lysed for 2 minutes (200 V), and the supernatant was collected. Combined with 40 μL of Glutathione Sepharose 4B (GE Company) gel beads (beads) used for binding GST fusion protein, the binding condition was 4 ° C for 3 h; after the binding was completed, unbound foreign proteins were eluted with PBS for 3 times, and then After resuspending the medium with 40 μL PBS, take 40 μL for electrophoresis. Add 5 μL of PreScissionprotease (PP enzyme, GE ...
Embodiment 3
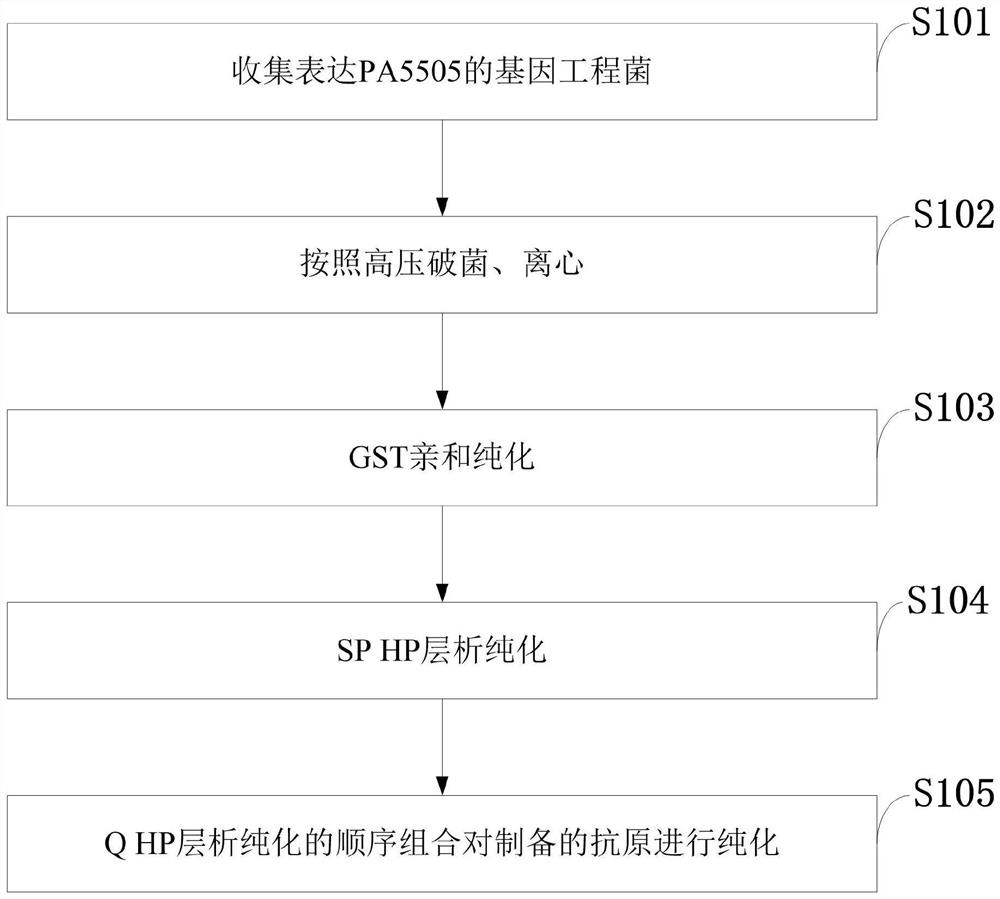
[0070] Example 3 Purification process research of PA5505
[0071] By exploring the purification conditions of PA5505, the process flow of the protein purification was finally determined, such as figure 1 As shown, the specific operation is as follows.
[0072] 1. Autoclave, centrifuge
[0073] The Escherichia coli engineering bacteria expressing PA5505 were subjected to high-density fermentation, and the bacterial cells were collected by centrifugation for future use.
[0074] Take about 300g of bacteria, mix and suspend in PBS buffer according to the weight:volume ratio of 1:5, and pre-cool at 4°C.
[0075] High-pressure homogenizer: Use distilled water to flush the pipeline of the high-pressure homogenizer, and turn on the low-temperature circulation system to pre-cool to 1-4°C for later use.
[0076] Add the pre-cooled suspended bacteria liquid to a high-pressure homogenizer, maintain the pressure at 60-80Mpa to break the bacteria 3-5 times, take a smear of the broken ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com