Purifying method of methicillin-resistant staphylococcus aureus MRSA recombinant protein vaccine I1C
A methicillin- and staphylococcus-resistant technology is applied in the field of biopharmaceuticals to achieve the effects of high humoral immune response, simple process and good immune protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 autoclave, centrifugation
[0032] The recombinant double-subunit genetic engineering protein I constructed by the applicant to express soluble methicillin-resistant Staphylococcus aureus 1 The Escherichia coli engineering bacteria of C (refer to the invention patent application of 201310021212.3, the antigen I 1 The nucleotide sequence of C is shown in SEQ ID NO: 1, and its amino acid sequence is shown in SEQ ID NO: 2). Through high-density fermentation, the expression rate of the target protein was 13%, and the cells were collected by centrifugation for later use.
[0033] 200-500 g of bacterial cells were mixed with PBS (10-20 mM, pH 7.0-7.5) buffer solution according to the weight: volume ratio of 1:10, and pre-cooled at 4°C.
[0034] High-pressure homogenizer: Use distilled water to flush the pipeline of the high-pressure homogenizer, and turn on the low-temperature circulation system to pre-cool to 1-4°C for later use.
[0035] Add the pre-cooled su...
Embodiment 2
[0037] Embodiment 2 ammonium sulfate step-by-step precipitation, redissolving:
[0038] Under the condition of stirring at 4°C, slowly add ammonium sulfate with a final concentration of 30% to the supernatant, stir for more than half an hour, centrifuge at 10000-15000g for more than 20 minutes, and collect the supernatant; continue to slowly add ammonium sulfate with a final concentration of 40% to the supernatant Ammonium sulfate, stirred for more than half an hour, centrifuged at 10000-15000g for more than 20 minutes at high speed, and collected the precipitate;
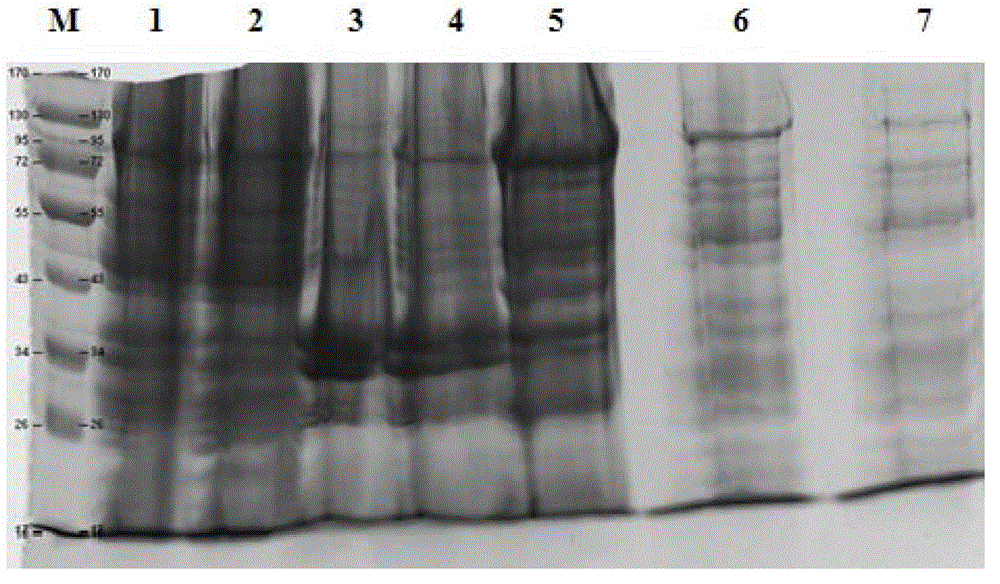
[0039] Precipitation redissolution: Weigh the wet weight of the precipitate and add buffer A (10-20mM Na 2 HPO 4 -NaH 2 PO 4 , 0.5M NaCl, 2mM EDTA, 0.5% Poloxamer188, pH7.0-7.5), stirred and mixed for 10-15 minutes, centrifuged at 10000-15000g for more than 20 minutes, and collected the supernatant; the electrophoresis results of the reconstituted samples were as follows: figure 1 As shown, I 1 The purity of p...
Embodiment 3G
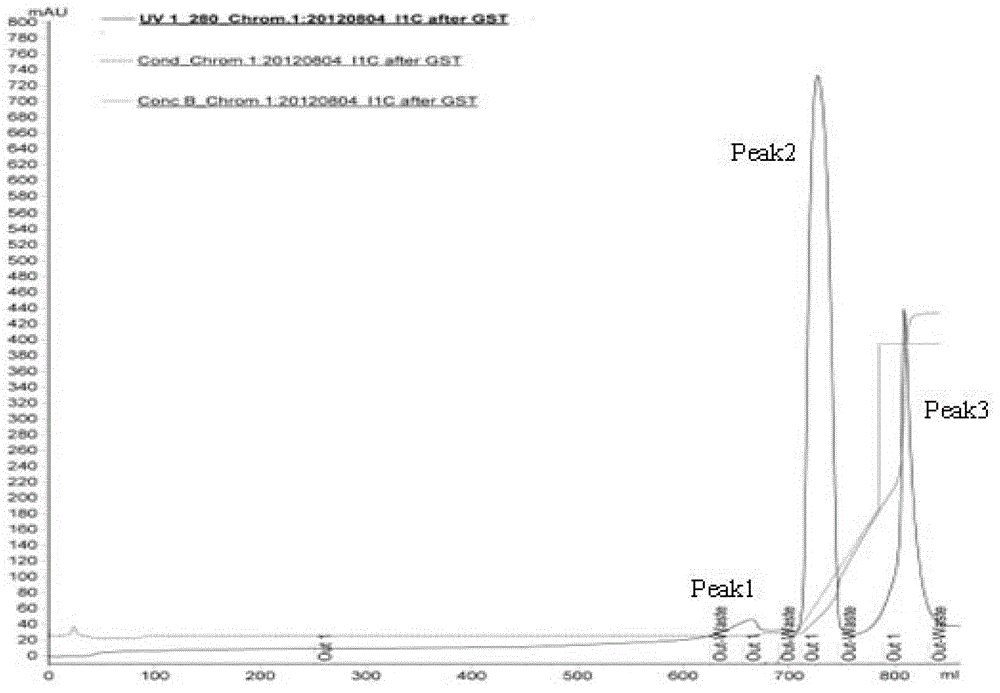
[0040] Embodiment 3 GST affinity purification:
[0041] Choose GST affinity chromatography filler for preliminary purification, GST affinity filler is one of GST-Sepharose4B, GST-Sepharose6B, GST-Sepharose FastFlow, GST-Sepharose HP, and the amount of filler per 100g wet weight of broken bacteria is 100ml.
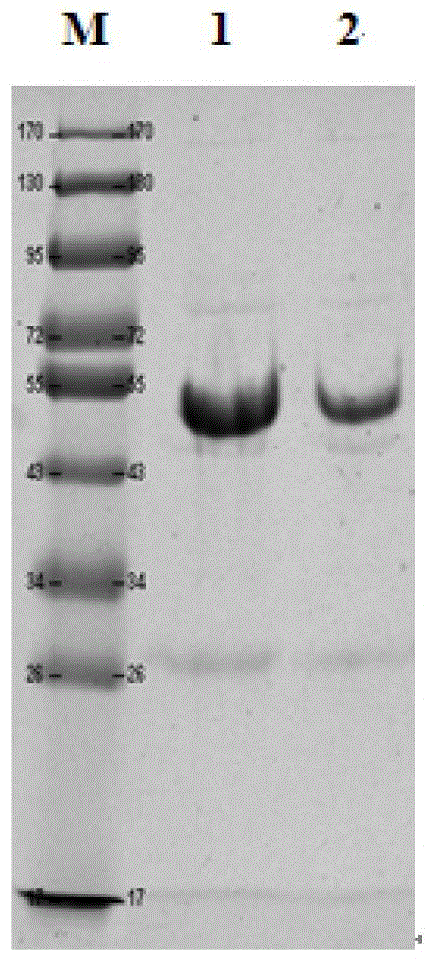
[0042] Prescission Protease (PP enzyme) for enzyme digestion and elution: the PP enzyme used has a GST tag to facilitate the removal of PP enzyme and obtain I 1 C target protein, electrophoresis results are as follows figure 2 shown. After GST primary purification, the purity of the target protein is further improved, reaching about 85%, and further purification is still needed to remove trace impurities.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com