Metal aluminum nano adjuvant, preparation method thereof, vaccine composition, preparation method of vaccine composition and application of metal aluminum nano particle
A vaccine composition, metal aluminum technology, applied in the directions of vaccines, drug combinations, DNA/RNA vaccination, etc., can solve the problems of low induction efficiency, and achieve the enhancement of humoral immune response, enhancement of cellular immune response, stability and safety. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 Preparation of metal aluminum nanoparticles by electric explosion method
[0118]In an argon environment, fix an aluminum wire with a diameter of 0.2mm and a purity greater than or equal to 95% between high-voltage electrodes, and use a mechanical device to continuously supply power. The power supply capacitance is 96μF, and the electrode discharge voltage is 4.0-4.4kV. Under high-density current, the aluminum wire is heated and radially expanded to vaporize the aluminum wire to form aluminum vapor, which is then condensed to form aluminum particles with an average particle size of 139.76±42.81nm, which is carried out in a collector filled with argon. collect. (See literature: Passivation Process for Superfine Aluminum Powders Obtained by Electrical Explosion of Wires. Appl. Sur. Sci. 2003, 211, 57–67.)
Embodiment 2
[0119] Example 2 Preparation of metal aluminum nanoparticles by ligand regulation method
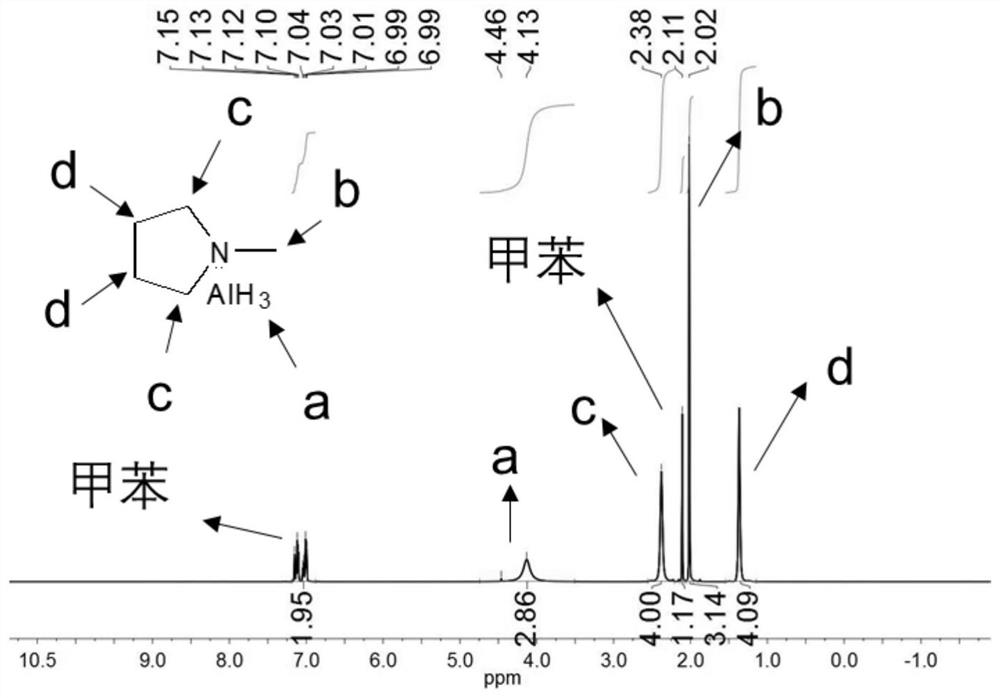
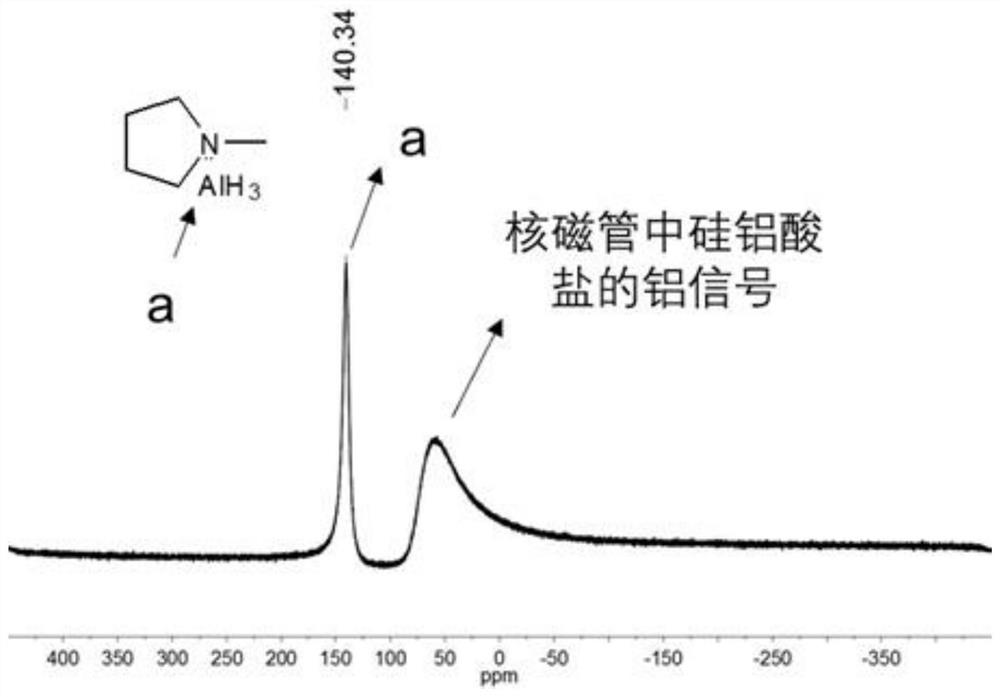
[0120] (1) Precursor H 3 Synthesis and Characterization of Al(1-MP)
[0121] In a glove box (with both oxygen and water content below 1 ppm), 3.748 g of lithium aluminum hydride and 4.132 g of aluminum chloride were added to a flask containing 45 mL of anhydrous toluene. Under vigorous stirring (stirring speed: 800 rpm), 11.65 mL of 1-methylpyrrolidine (1-methylpyrrolidine) was added dropwise. Wherein, the molar ratio of lithium aluminum hydride, aluminum chloride, and 1-methylpyrrolidine is 3:1:1. After overnight reaction at room temperature, the reaction solution was filtered through a funnel to remove solid impurities. For further purification, the obtained filtrate was filtered again with an organic phase filter membrane with a pore size of 0.22 μm, and the obtained filtrate was H 3 Al(1-MP) (structural formula is Yield is 90%) in toluene solution, and low-temperature preservat...
Embodiment 3
[0136] Vaccine AlNPs-OVA was prepared by mixing electroexplosive metallic aluminum nanoparticles with OVA. Specific steps are as follows:
[0137] (1) The electroexplosive metal aluminum nanoparticles prepared in Example 1 are made into a solution of 0.1-5.0 mg / mL (using DMF as a solvent). minute.
[0138] (2) Prepare ovalbumin OVA PBS solution with a concentration of 0.1-5.0 mg / mL. In this implementation, the concentration of ovalbumin OVA is 3.0 mg / mL.
[0139] (3) Mix the PBS solution of ovalbumin OVA and the DMF solution of nano-aluminum particles according to the volume of 1:1, ultrasonically disperse for half an hour, transfer to a shaker, and shake at room temperature at 320rpm for 12 hours. After stopping the shaking, centrifuge at 12000rpm for 10 minutes, remove the supernatant and freeze-dry the sample to remove the remaining small amount of solvent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com