GITR binding molecules and uses therefor
A technique of combining molecules and compositions, which is applied in the field of GITR binding molecules and their uses, and can solve problems such as inability to protect cells from apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0386] Example 1: Isolation and purification of 6C8
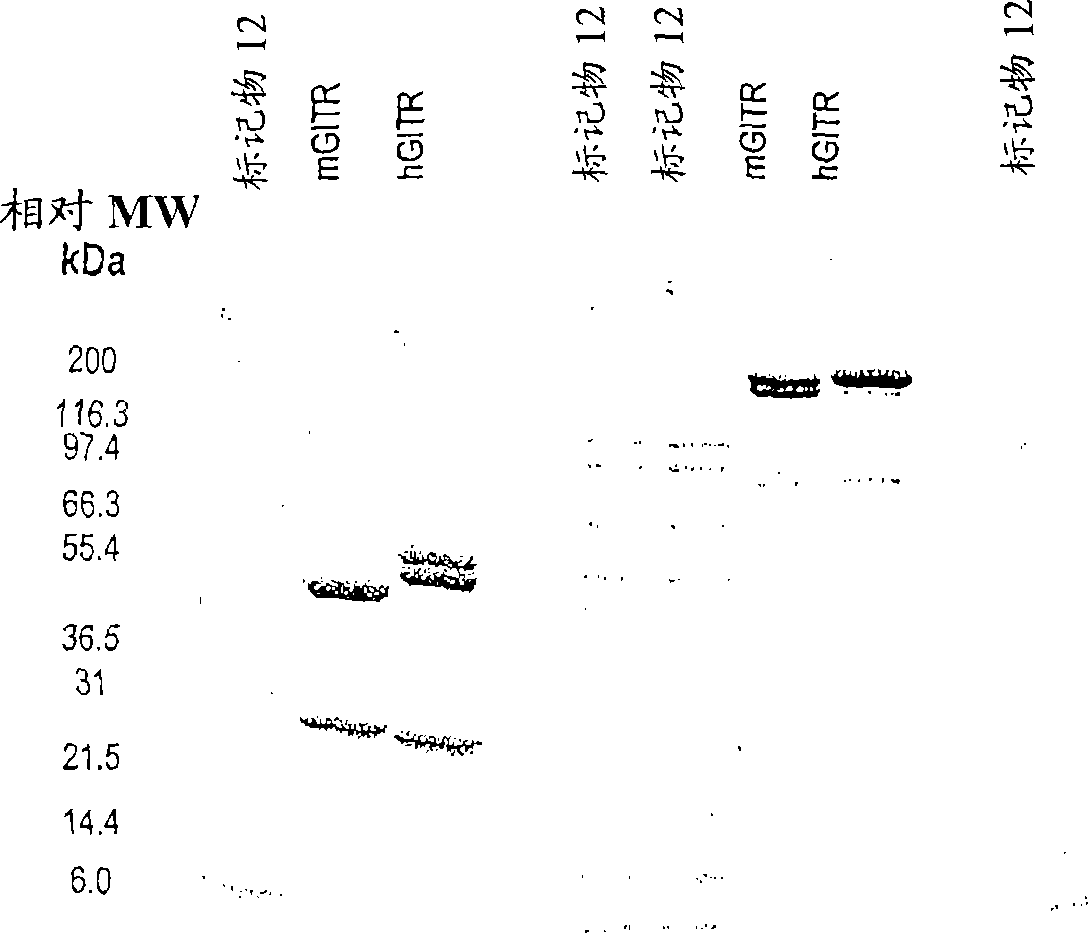
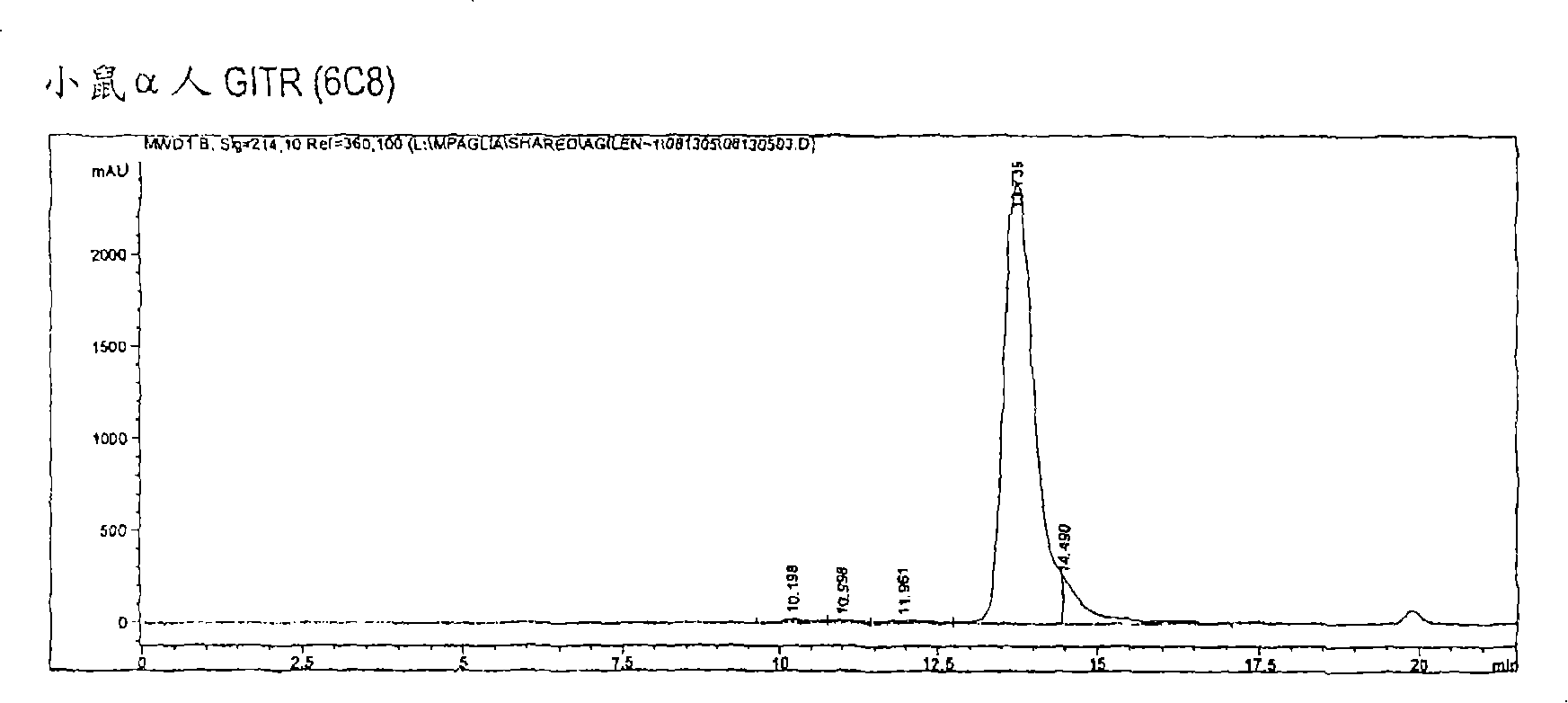
[0387] The 6C8 antibody is IgG2b, kappa type. Purification of this antibody revealed the presence of two heavy chains ( figure 1 ). This can be due to taking one of the two glycosylations or contamination with another antibody (Ab). Size exclusion chromatography showed only one peak ( figure 2 ).
[0388] The 6C8 antibody was purified as follows:
[0389] 1. Wash 20ml protein G (Pharmacia HR10 / 30) with 5 column volumes of dPBS
[0390] 2. Load 1L (1st round) or 2L (2nd round) hGITR (6C8) supernatant
[0391] 3. Wash with 10 column volumes of dPBS
[0392] 4. Elute directly into 1M Tris (20-25% v; v) with 100 mM citrate, pH 2.8
[0393] 5. Strip with 100mM citrate (pH 2.8), 0.3M NaCl
Embodiment 2
[0394] Example 2: Identification of 6C8
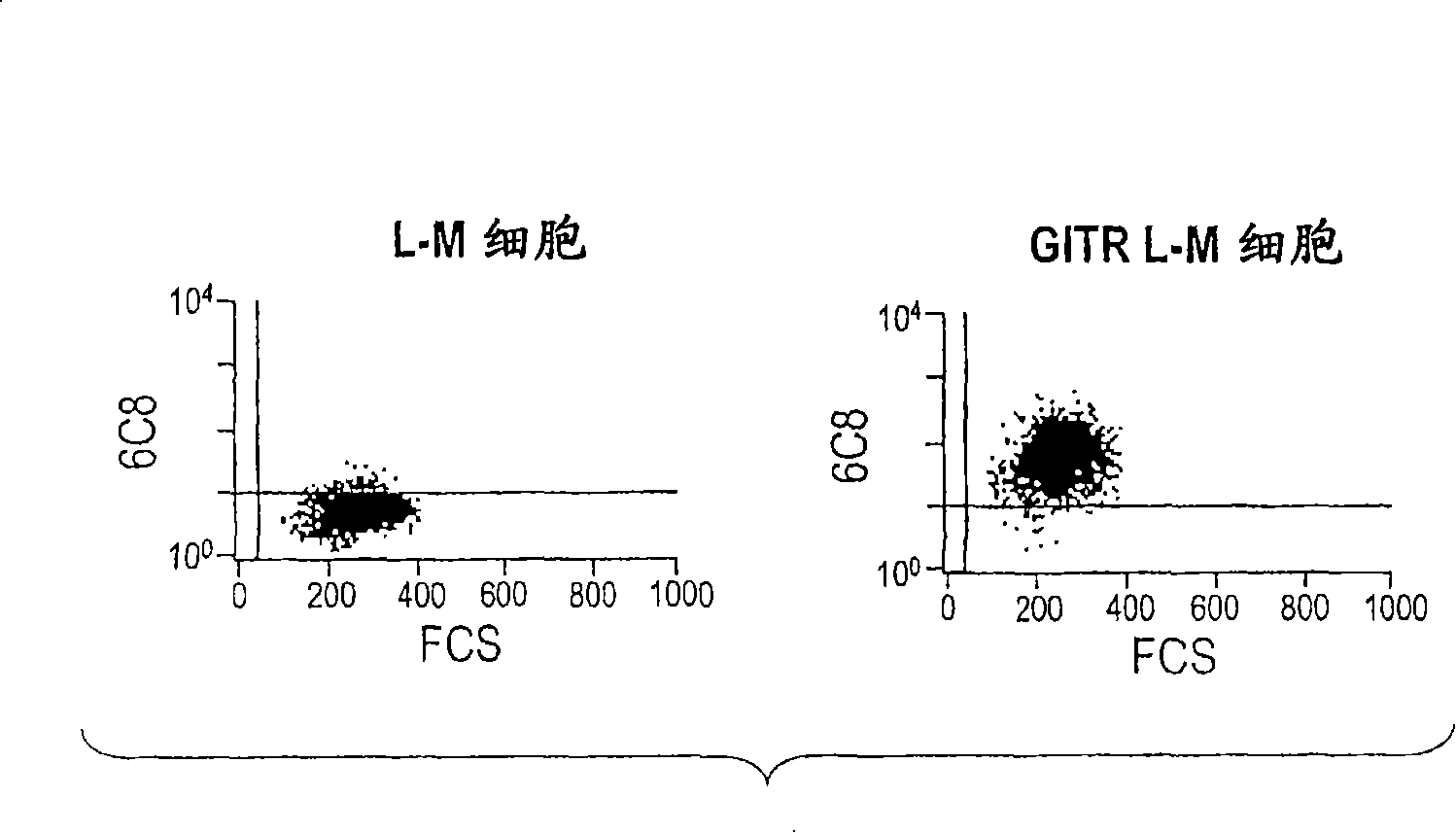
[0395] 6C8 antibody and cells transfected with GITR-L-M ( image 3 ) and activated PBL ( Figure 4 ) combined. Saturation curves of biotin-labeled anti-GITR on activated lymphocytes suggest good relative affinity ( Figure 5 ).
[0396] The 6C8 antibody has co-stimulatory activity on T lymphocytes activated with suboptimal anti-CD3 ( Figure 6 ). Costimulation of this antibody did not reach the same level as CD28, but was comparable to the anti-GITR commercial product (R&D).
[0397] The 6C8 antibody did not induce apoptosis in activated lymphocytes (Figure 7). Lymphocytes were activated with PHA, and antibodies were added 3 days later. Compared to YTH 655, an anti-human CD2 known to induce apoptosis in activated lymphocytes, 6C8 did not increase apoptosis in activated T lymphocytes.
[0398] 6C8 antibody did not block the primary mixed lymphocyte reaction (MLR) ( Figure 8 ). TRX1 (anti-human CD4) was used as a positive cont...
Embodiment 3
[0399] Example 3: 6C8 antibody counteracts the inhibitory effect of regulatory T cells on T effector cells
[0400] Antibody 6C8 was able to block the suppressive effect induced by regulatory T cells ( Figure 9 ). CD4+ / CD25+ cells were added to CD4+ / CD25- cells at different ratios. The cells were stimulated with anti-CD3 and anti-CD28 bound to microwell plates. When the ratio is 1:1, CD4+ / CD25+ cells are able to eliminate the proliferation of CD4+ / CD25- cells. Addition of 6C8 to the cultures blocked this inhibitory effect in a dose-dependent manner.
[0401] When T cells were stimulated by anti-CD3 alone (not co-stimulation with anti-CD28), CD4+ / CD25+ cells were added to CD4+ / CD25- cells, no inhibitory effect was observed, and indeed the anti-GITR antibody was has a weak co-stimulatory effect ( Figure 10 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com