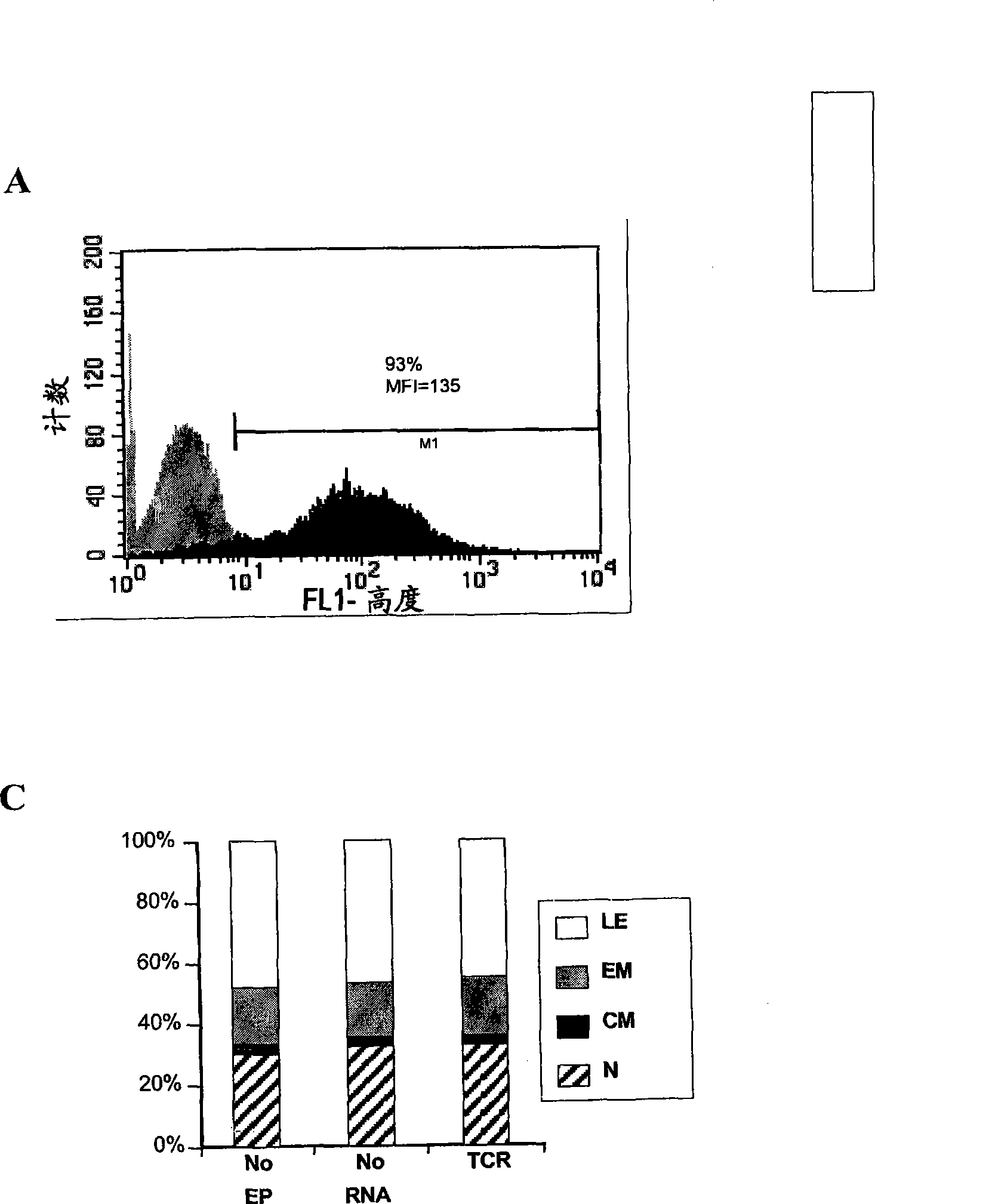
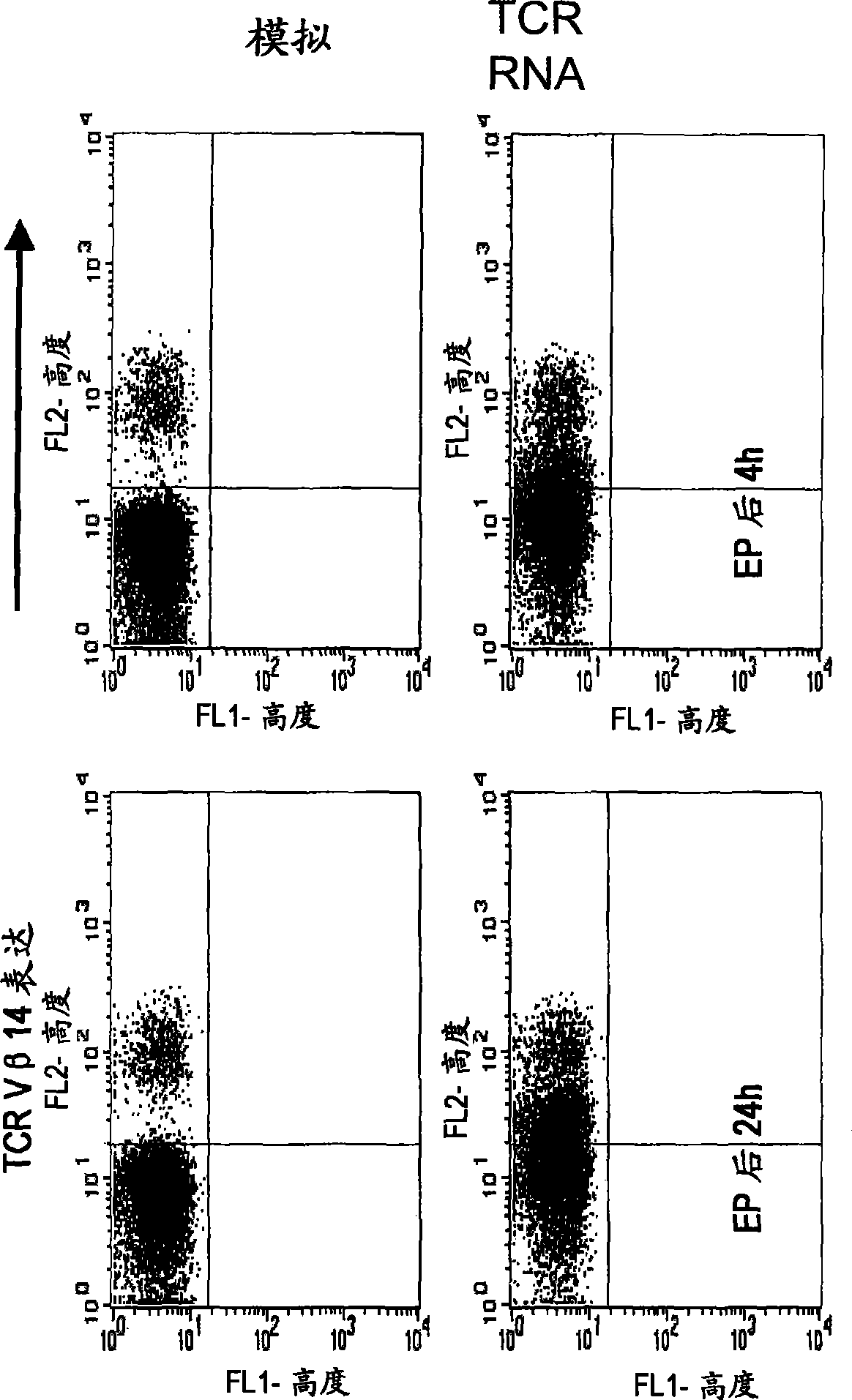
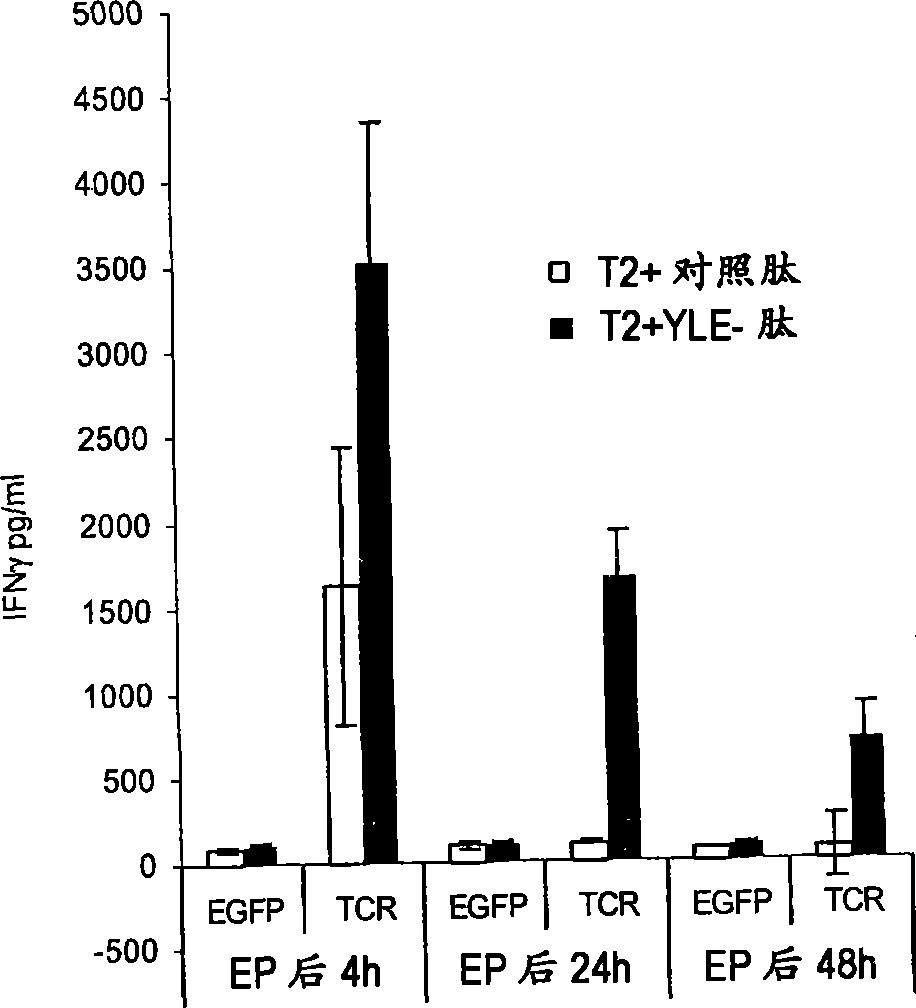
Methods for generating antigen-specific effector T cells
A specific, cellular technology that is used in biochemical equipment and methods, receptors/cell surface antigens/cell surface determinants, other methods of inserting foreign genetic material, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] While the making and using of various embodiments of the invention are described in detail below, it should be appreciated that the invention provides many advantageous inventive concepts that can be implemented in a wide variety of specific contexts. The specific embodiments described herein are merely illustrative of specific ways to make and use the invention, and do not limit the scope of the invention.
[0027] To facilitate the understanding of the present invention, terms defined herein have meanings commonly understood by those skilled in the art related to the present invention. Words such as "a", "an" and "the" do not denote only a single individual, but include the general class to which particular embodiments may be used for illustration. The terminology herein is used to describe particular embodiments of the invention, but their usage does not delimit the invention unless stated in the specification. For example, the term "a cell" includes a plurality of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com