Composition comprising a tramadol material and acetaminophen and its use
a technology of acetaminophen and tramadol, which is applied in the direction of drug compositions, biocide, amide active ingredients, etc., can solve the problems of undesirable side effects, hot flushes and sweating, and certain side effects of tramadol, and achieve synergistic analgesic effects and reduce the number and degree of side effects of each
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Combined Doses of Tramadol and APAP
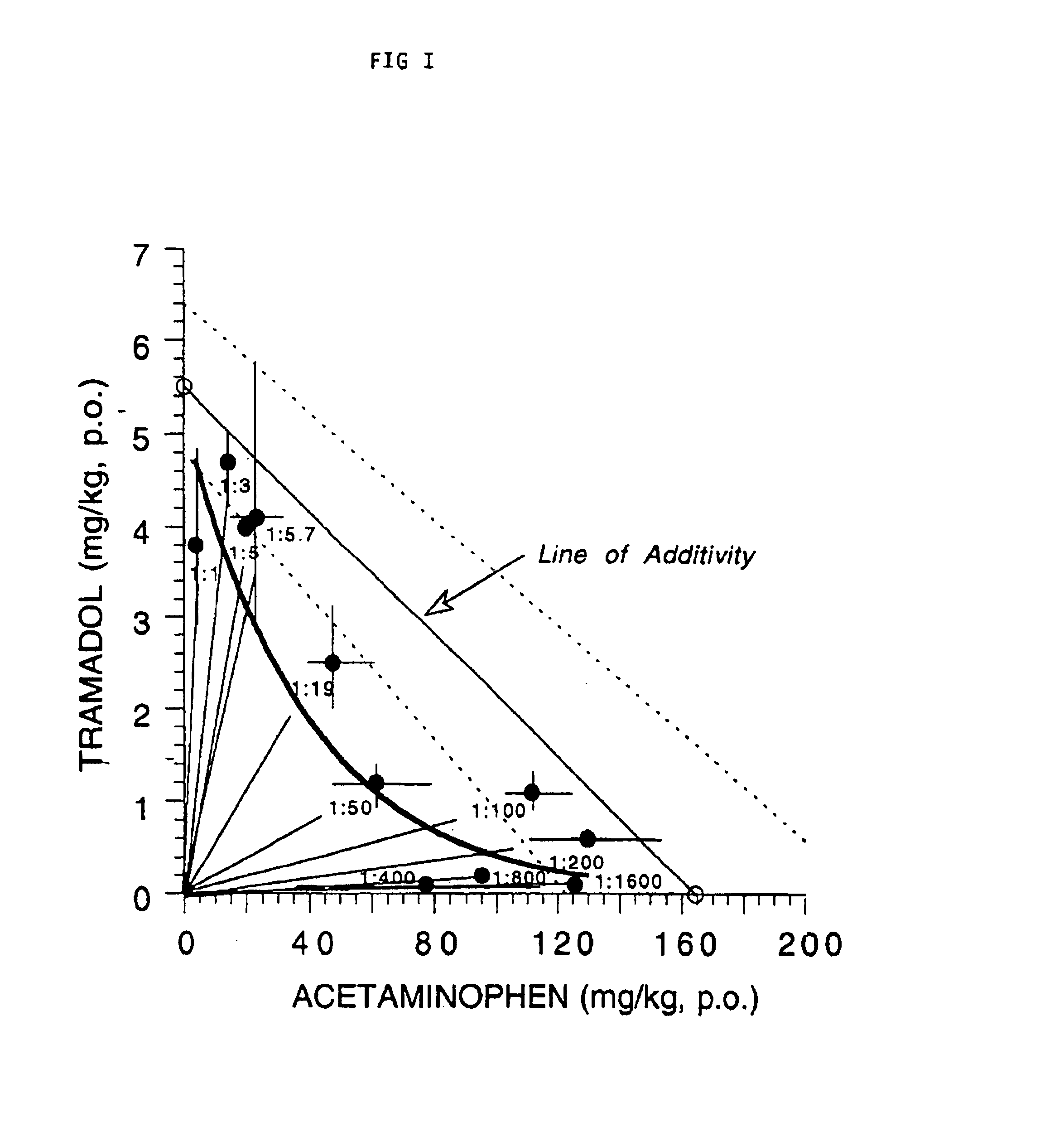
[0019]The preparation of different ratios of a tramadol / APAP combination is effected by first preparing a stock solution of tramadol having a concentration expressed in mgdrugs per 10 mL of distilled water. For example, 8 mg of tramadol as the free base is dissolved per 10 mL of water to yield the highest dose of tramadol stock solution. The stock solution of the tramadol is then diluted with a sufficient amount of distilled water to prepare the lower doses of the tramadol per 10 mL of distilled water. The combinations are then made by adding 10 mL of each dilution to the appropriate mg of APAP to achieve the desired ratio of tramadol to APAP. For the 1:50 example: 400 mg of APAP as the free base is suspended with 10 mL of the 8 mg tramadol solution and 2 drops of TWEEN 80, a pharmacological dispersant, manufactured by Fisher Scientific Company, to yield the 1:50 ratio, i.e. (8 mg:400 mg) combination per 10 mL of water. Each rati...
example 2
Preparation of the Combined Doses of Tramadol N-oxide and APAP
[0020]First, tramadol N-oxide was prepared as set forth hereinafter. Tramadol hydrochloride (0.5 mol) was converted to its free base in basified water (pH>9) and then extracted with ether. The ether was evaporated to yield the crystalline hydrate of tramadol. The solid was then heated with steam under a high vacuum to remove as much water as possible to yield 131.5 g of material. The material was dissolved in methanol (500 mL) and 65 g of 30% H2O2 was added. The solution was stirred for 3 hours and then an additional 65 g of the 30% H2O2 was added. The reaction was stirred for 2.5 days at room temperature. Approximately 10 mg of PtO2 on carbon (use of Pt / C is suggested for its ease of removal) was added to the reaction mixture, and very gentle foaming took place. An additional 10 mg of PtO2 was added and the reaction mixture was stirred overnight and then filtered thru a filter aid. The filtrate was concentrated under vac...
example 3
(−) and (+) Enantiomers of O-Desmethyl Tramadol: Their Syntheses and the Preparation of Doses of O-Desmethyl Tramadol-with APAP
[0023]First, O-desmethyl tramadol was prepared as set forth hereinafter. Diethylene glycol (125 mL) was added with cooling to potassium hydride (9.5 g) with the temperature being maintained at D25=−32.9 (C=1, EtOH).
[0024]C15H23NO2.HCl Theor.: C, 63.04; H, 8.46; N, 4.90 Found: C, 63.00; H, 8.51; N, 4.94
[0025]To prepare the (+) enantiomer of the title compound, the reaction was run under the same conditions except that (+)-tramadol as the free base was used instead of the (−)-tramadol to yield 2.8 g of the (+) enantiomer of O-desmethyl tramadol (mp. 242°-3° C.) [α]D25=+32.2 (C=1, EtOH).
[0026]C15H23NO2.HCl Theor.: C, 63.04; H, 8.46; N, 4.90 Found: C, 63.14; H, 8.49; N, 4.86
[0027]The preparation of different ratios of a O-desmethyl / APAP combination is effected by first preparing a stock solution of O-desmethyl tramadol having a concentration expressed in mgdrugs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| vapor temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com