Patents
Literature
199 results about "Opioid naive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of Opioid naive. Opioid naive means a patient who has not used opioids for more than seven consecutive days during the previous 30 days.
Formulation for intranasal administration
InactiveUS6017963AImprovement in flow property and dispensing accuracyPowder deliveryBiocideInhalationAnalgesic agents
A dosage form for intranasal and / or inhalation administration of an analgesic having low opioid receptor binding activity to a warm blooded animal, that includes a pharmaceutically effective amount of the analgesic. A preferred analgesic is tramadol, a tramadol and / or a pharmaceutically acceptable salt, metabolite or derivative thereof. Methods of making and using the formulation according to the invention are also provided.
Owner:EURO-CELTIQUE SA
Alcohol Resistant Dosage Forms
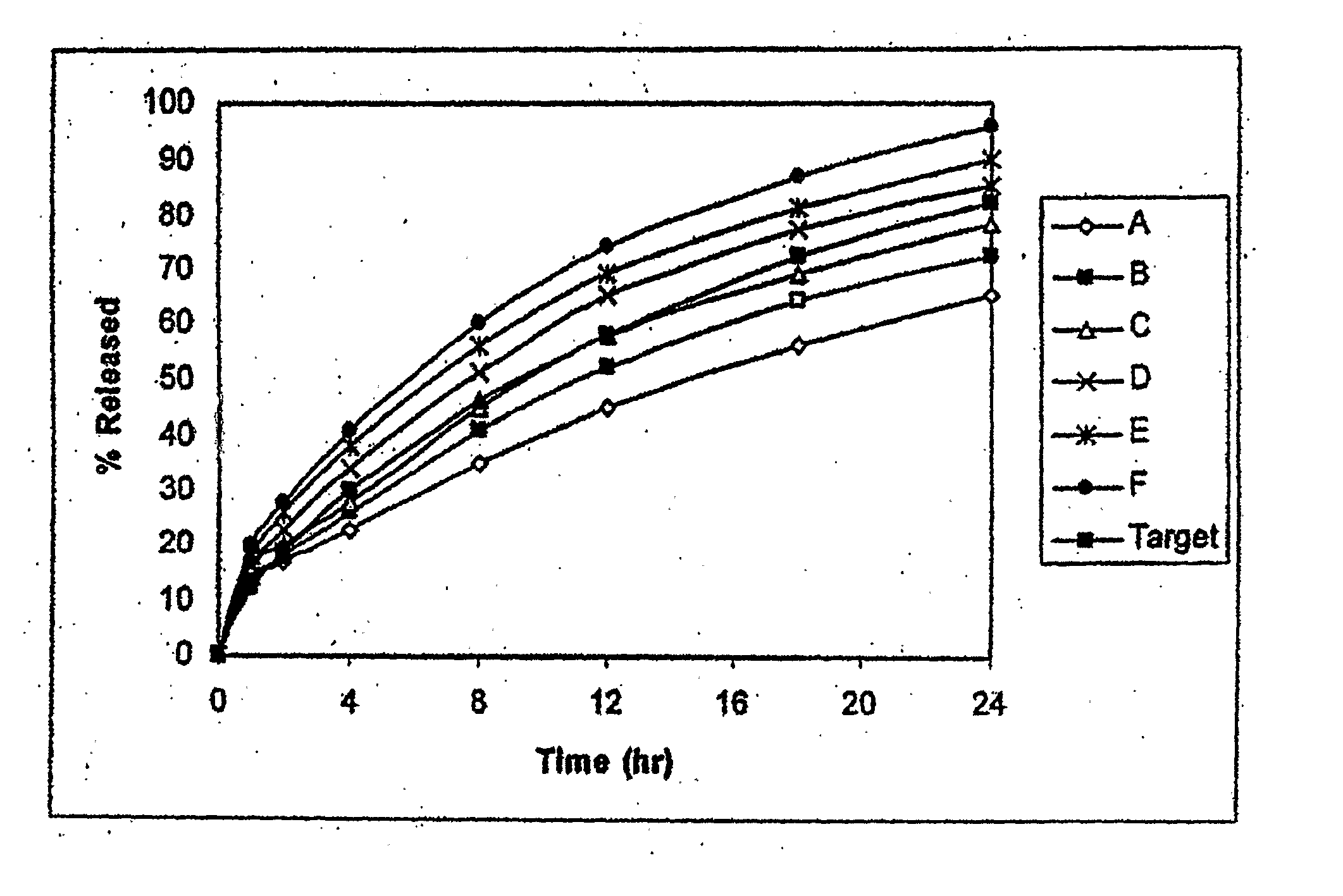
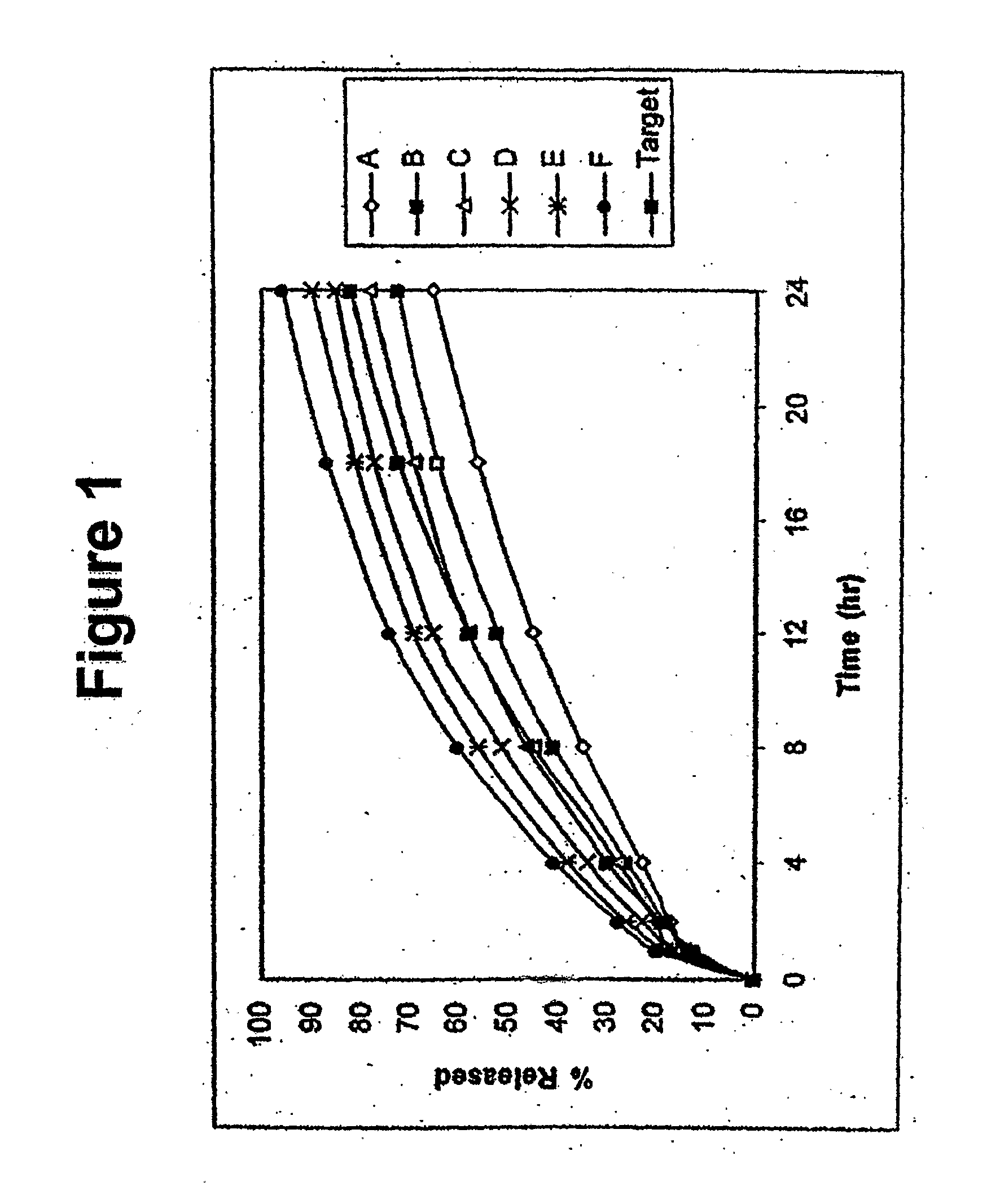
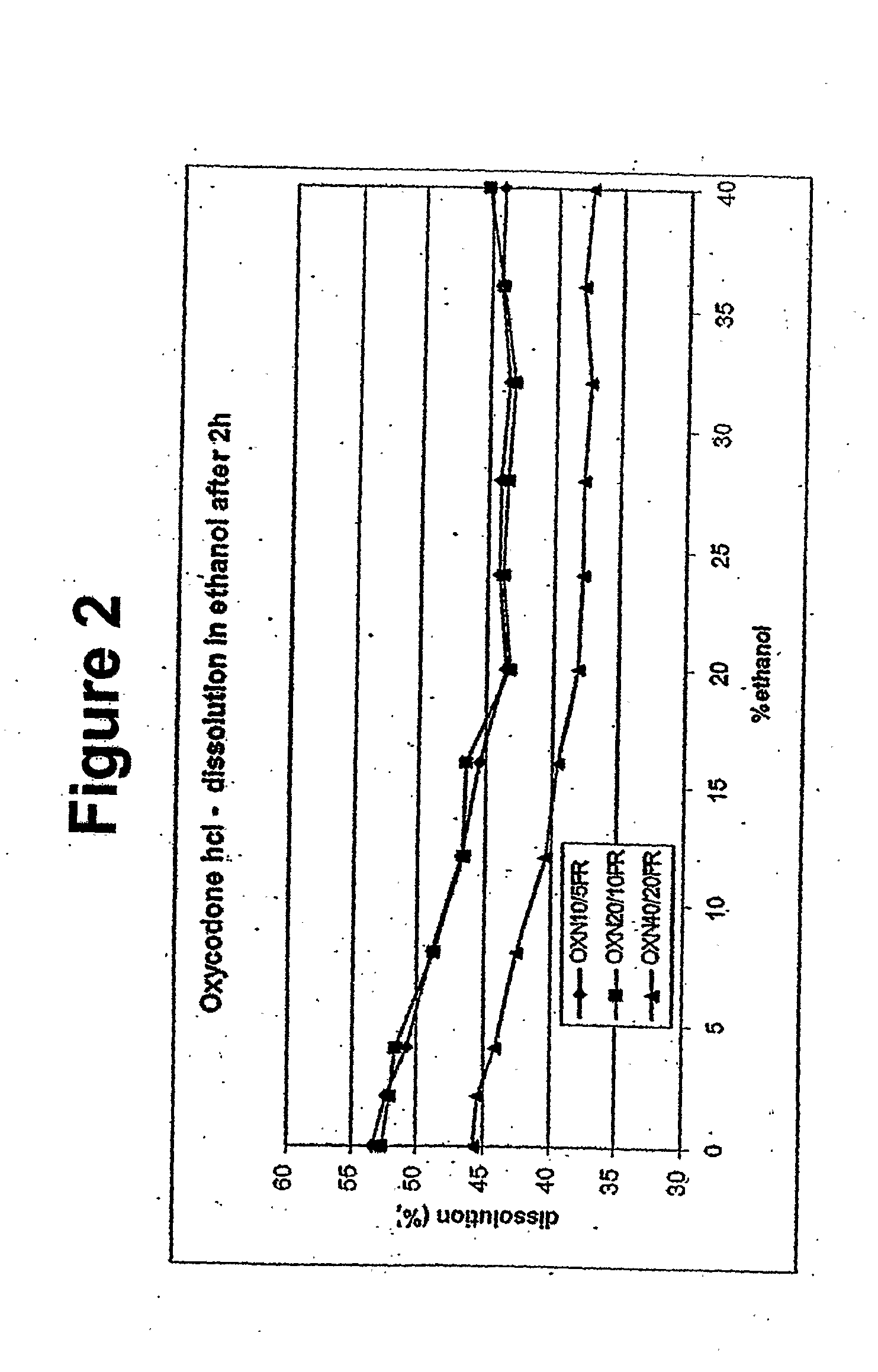
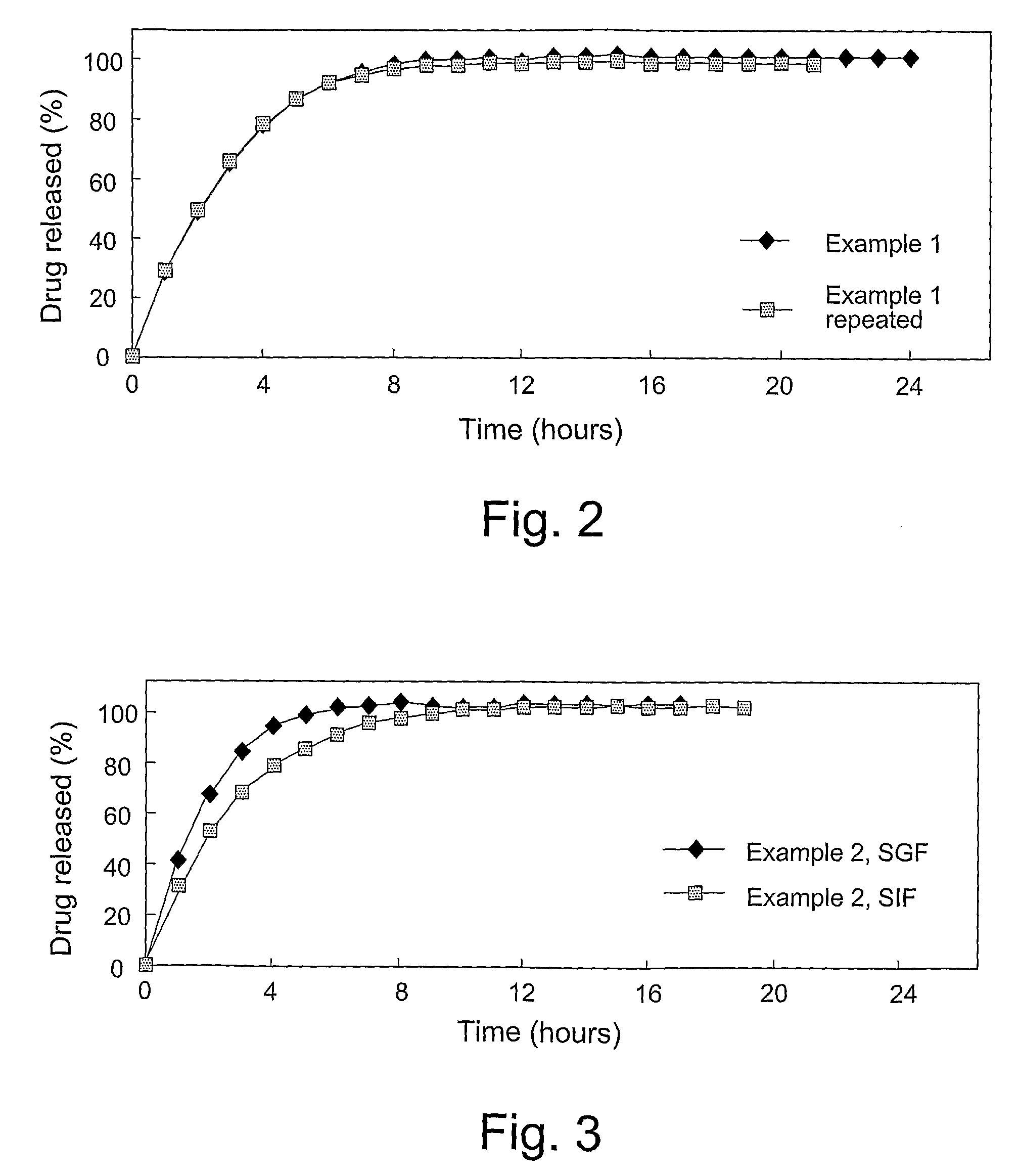
Disclosed in certain embodiments is a controlled release dosage form comprising a matrix comprising a pharmaceutically acceptable salt of an opioid analgesic in a controlled release material; wherein less than 25% of the opioid salt is released after 1 hour of in-vitro dissolution of the dosage form in 900 ml of Simulated Gastric Fluid with 20% ethanol using a USP Apparatus I (basket) apparatus at 100 rpm at 37 degrees C.°.
Owner:PURDUE PHARMA LP
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Multiparticulates
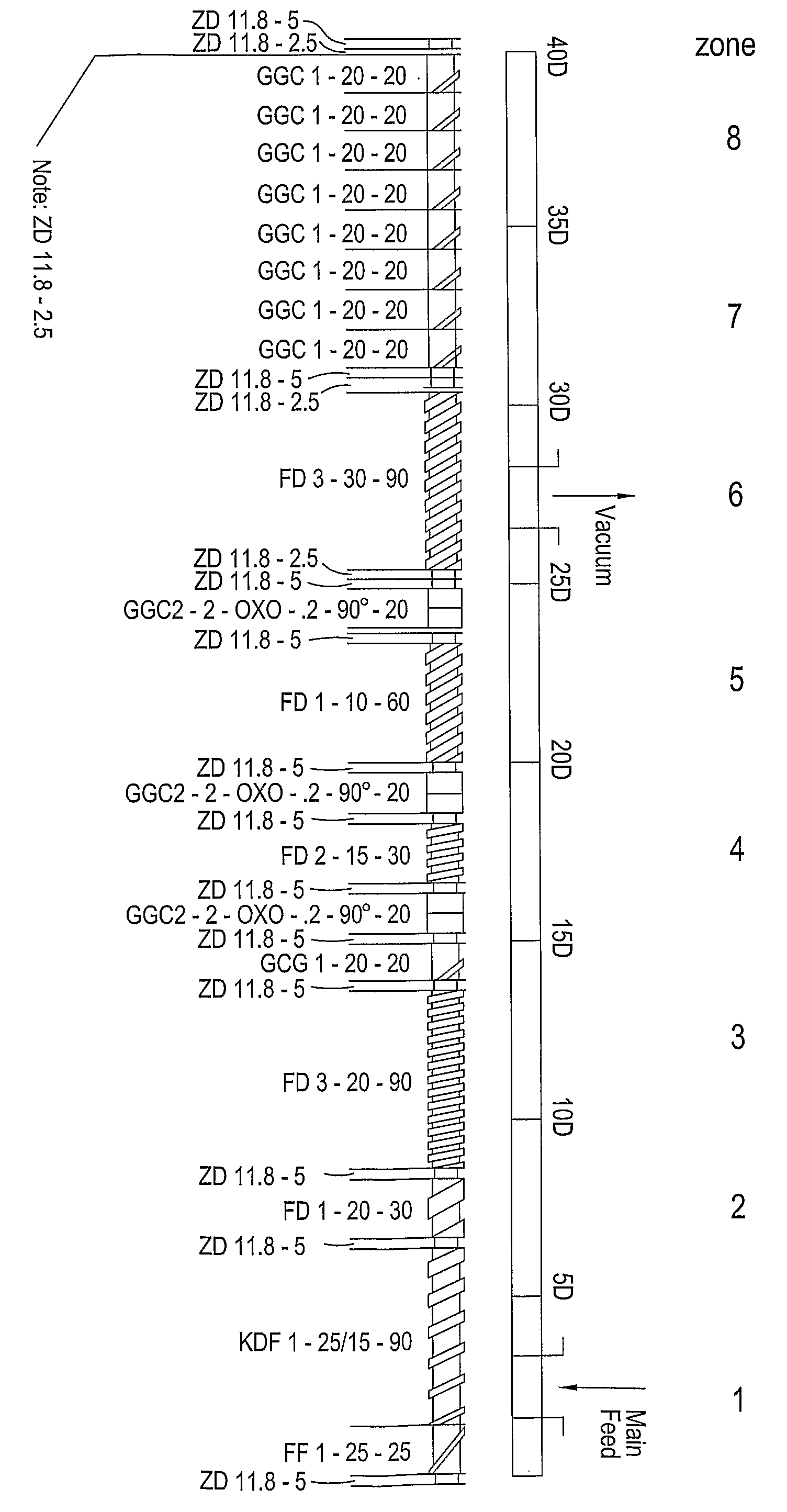
InactiveUS20080260815A1Reduce adhesionReduce the required powerOrganic active ingredientsPowder deliveryActive agentExcipient
Extrusion of a mix containing a pharmaceutically active agent can be achieved using a plasticising excipient in an amount sufficient to act as plasticiser and also act as lubricant, thereby avoiding the need for inclusion of a lubricant. The invention provides multiparticulates with controlled release properties, substantially free of lubricant. The present invention is preferably directed to extruded multiparticulates containing an opioid such as oxycodone, an ammonium methacrylate copolymer such as Eudragit® RSPO, a plasticising excipient such as preferably stearyl alcohol and a water permeability modifier such as preferably Eudragit® RLPO. The obtained multiparticulates show a release rate profile which is pH-independent.
Owner:EURO-CELTIQUE SA
Composition comprising a tramadol material and acetaminophen and its use
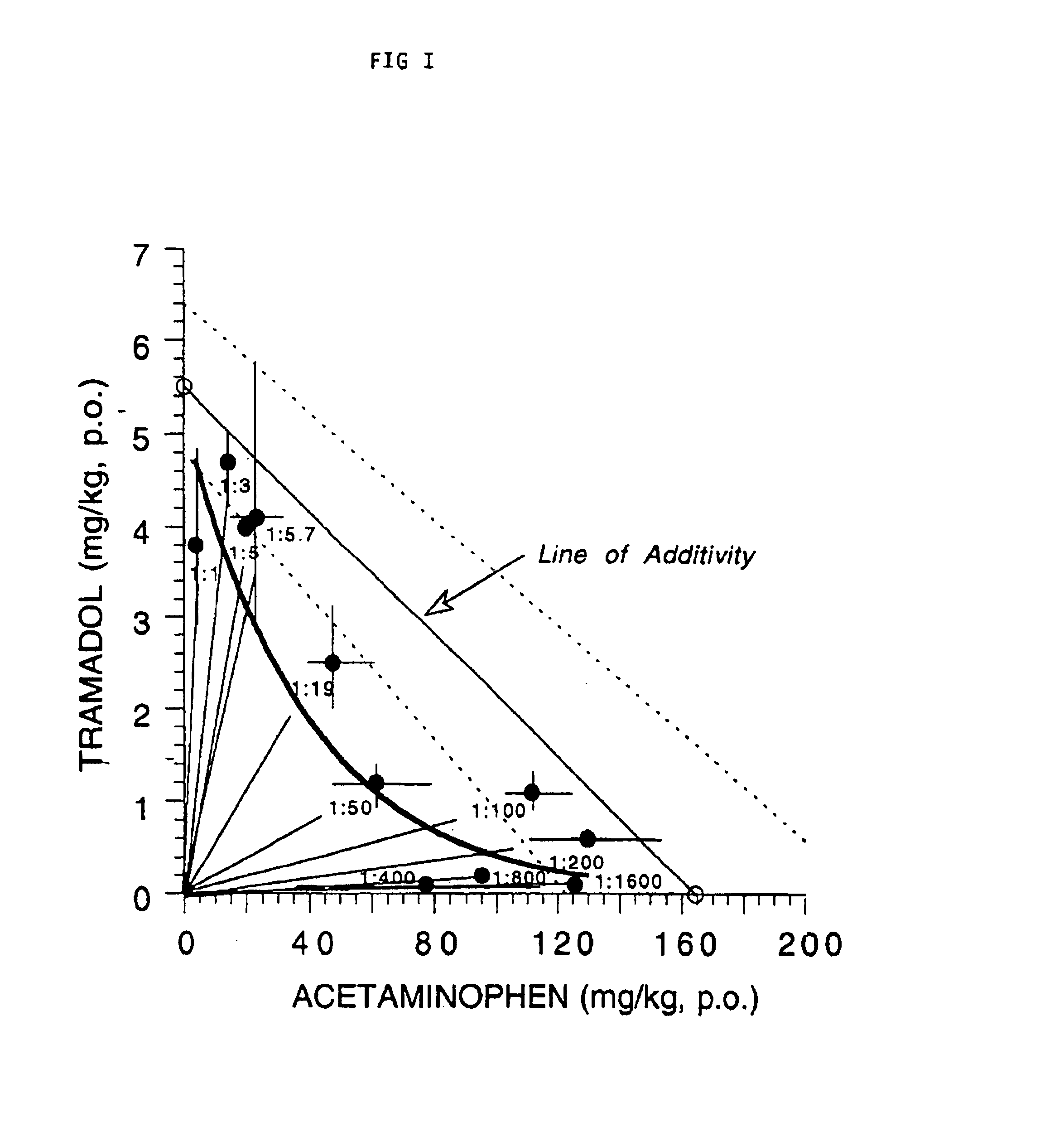
This invention relates to a composition comprising a tramadol material and acetaminophen, and its use. As used herein tramadol refers to various forms of tramadol. The compositions are pharmacologically useful in treating pain and tussive conditions. The compositions are also subject to less opioid side-effects such as abuse liability, tolerance, constipation and respiratory depression. Furthermore, where the components of the compositions are within certain ratios the pharmacological effects of the compositions are superadditive (synergistic).
Owner:ORTHO MCNEIL PHARM INC
Methods for the modulation of brain progestagen signaling in the prevention and treatment of neurological disorders and neurodegenerative diseases
InactiveUS20100028360A1Promoting neurodegenerationImprove cognitive abilityBiocideOrganic active ingredientsNeurogenesisPhysiology
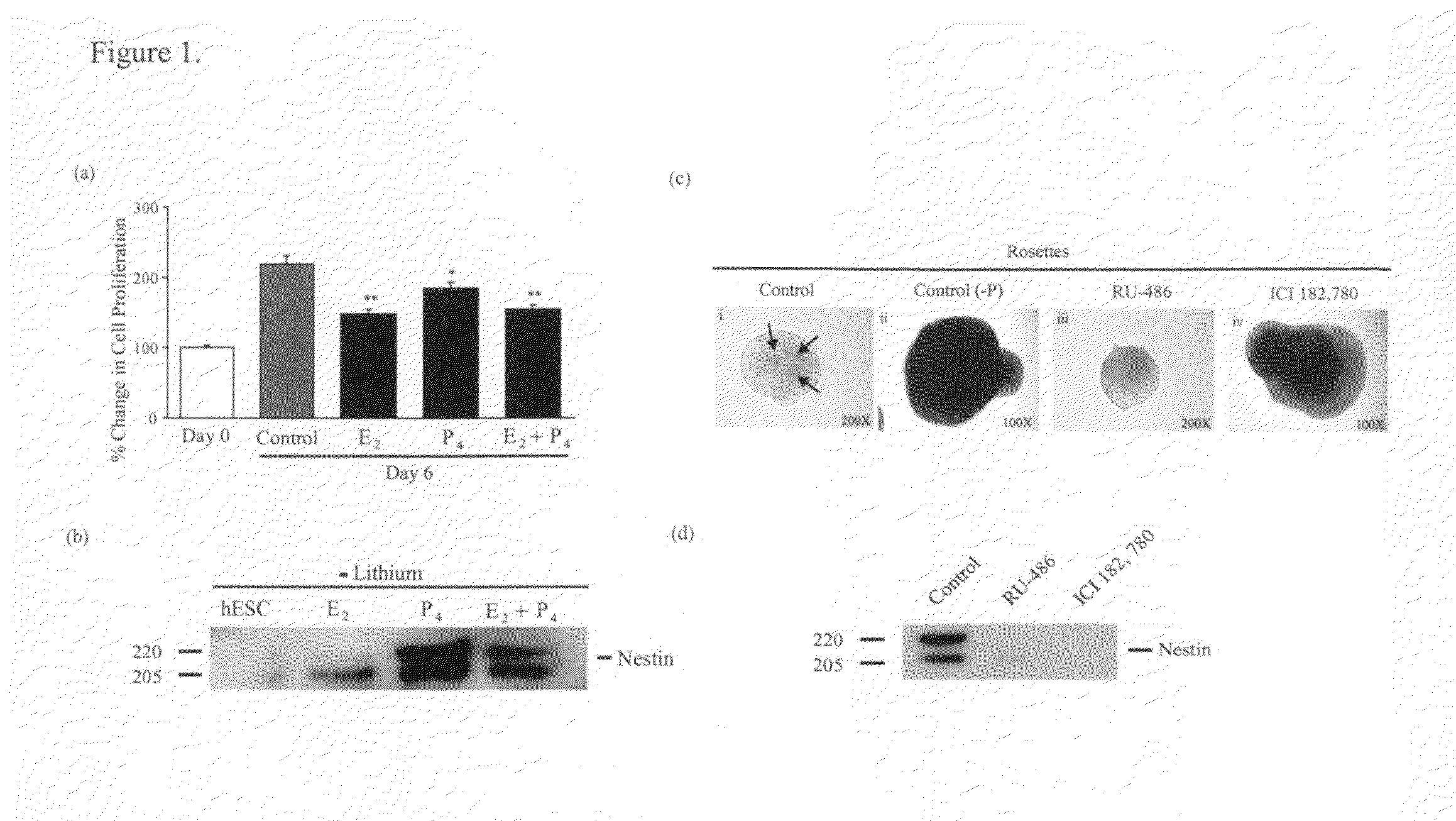
The present invention relates to methods for modulating progestagen signaling for treating neurological disorders or neurodegenerative disease, or preventing or delaying its onset in individuals deemed by competent observation and testing to be susceptible thereto. Progestagens can be administered to elevate serum and brain levels of progestagens and induce neurogenesis. Progestagen therapy may prevent some of the neurodegenerative and cognitive changes associated with developmental and aging associated neurological disorders and neurodegenerative diseases. Progestagen therapy together with suppression of GnRH, kisspeptin, LH and / or FSH signaling also may be used for treating neurological disorders or neurodegenerative diseases. The invention also relates to methods for inhibiting or delaying blastulation during embryogenesis, and neurogenesis during embryogenesis, fetal, neonatal, childhood, puberty or adult life. Blocking progestagen, estrogen and / or opioid signaling with receptor antagonists will inhibit neurogenesis. The invention also relates to using progestagens in vitro to induce neurogenesis in embryonic or adult stem cells.
Owner:ATWOOD CRAIG STEPHEN
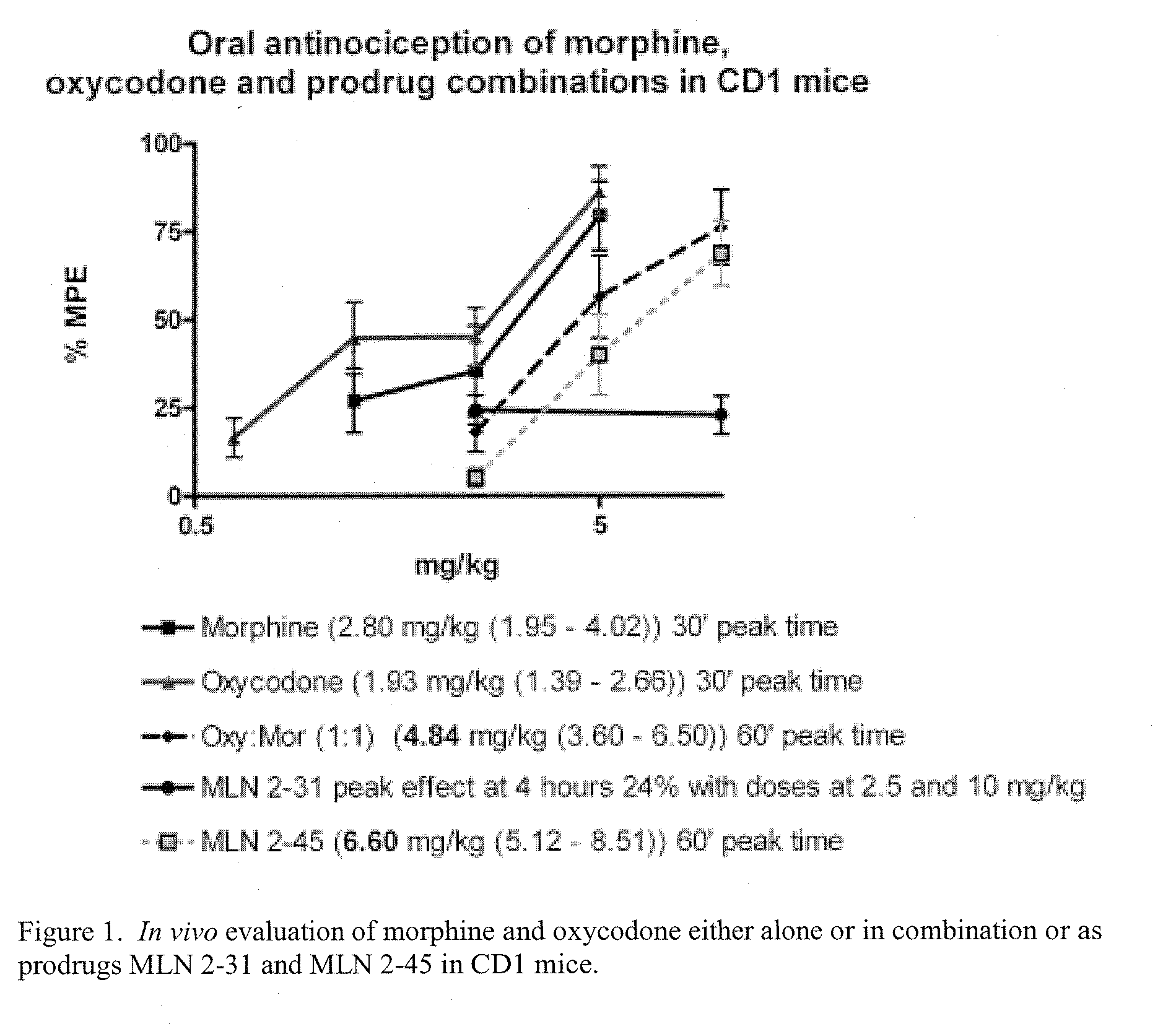
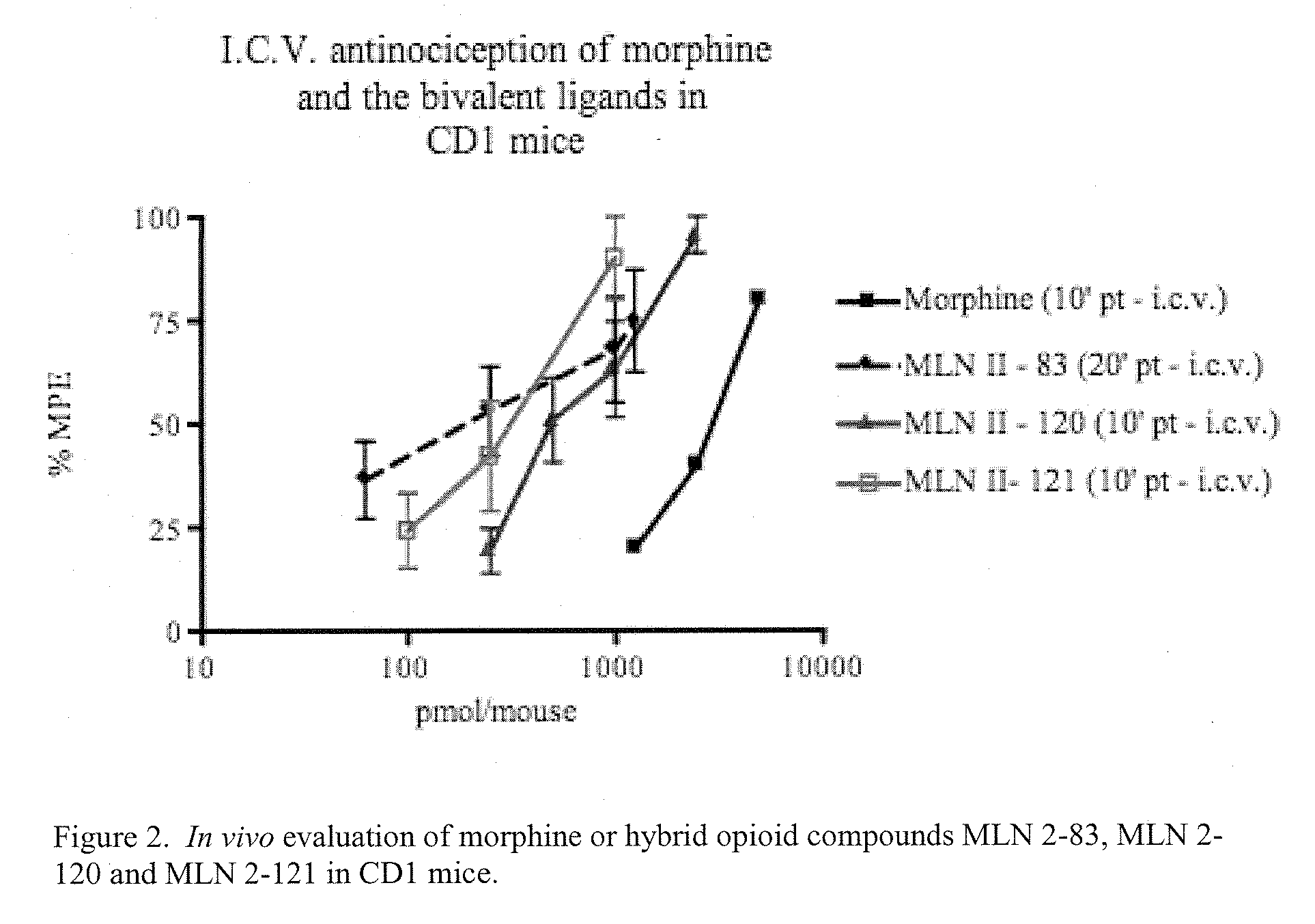
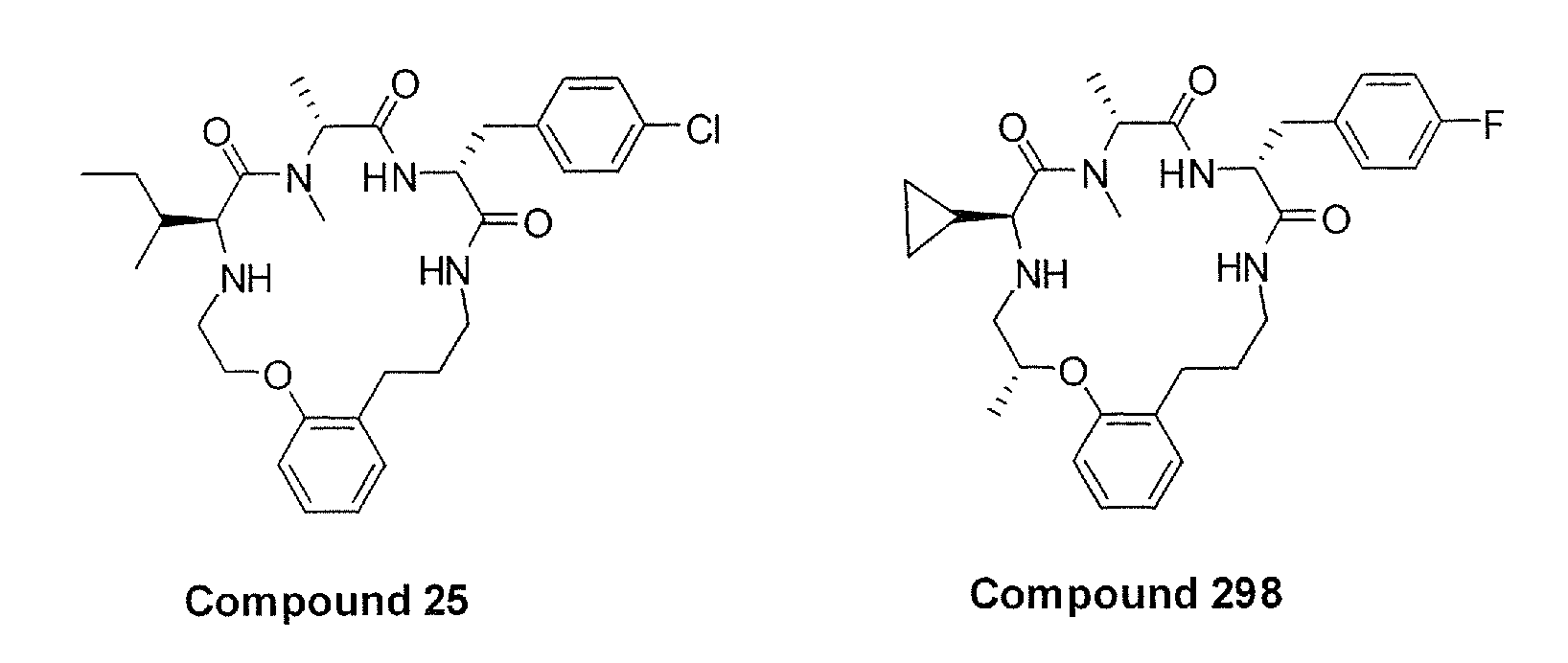
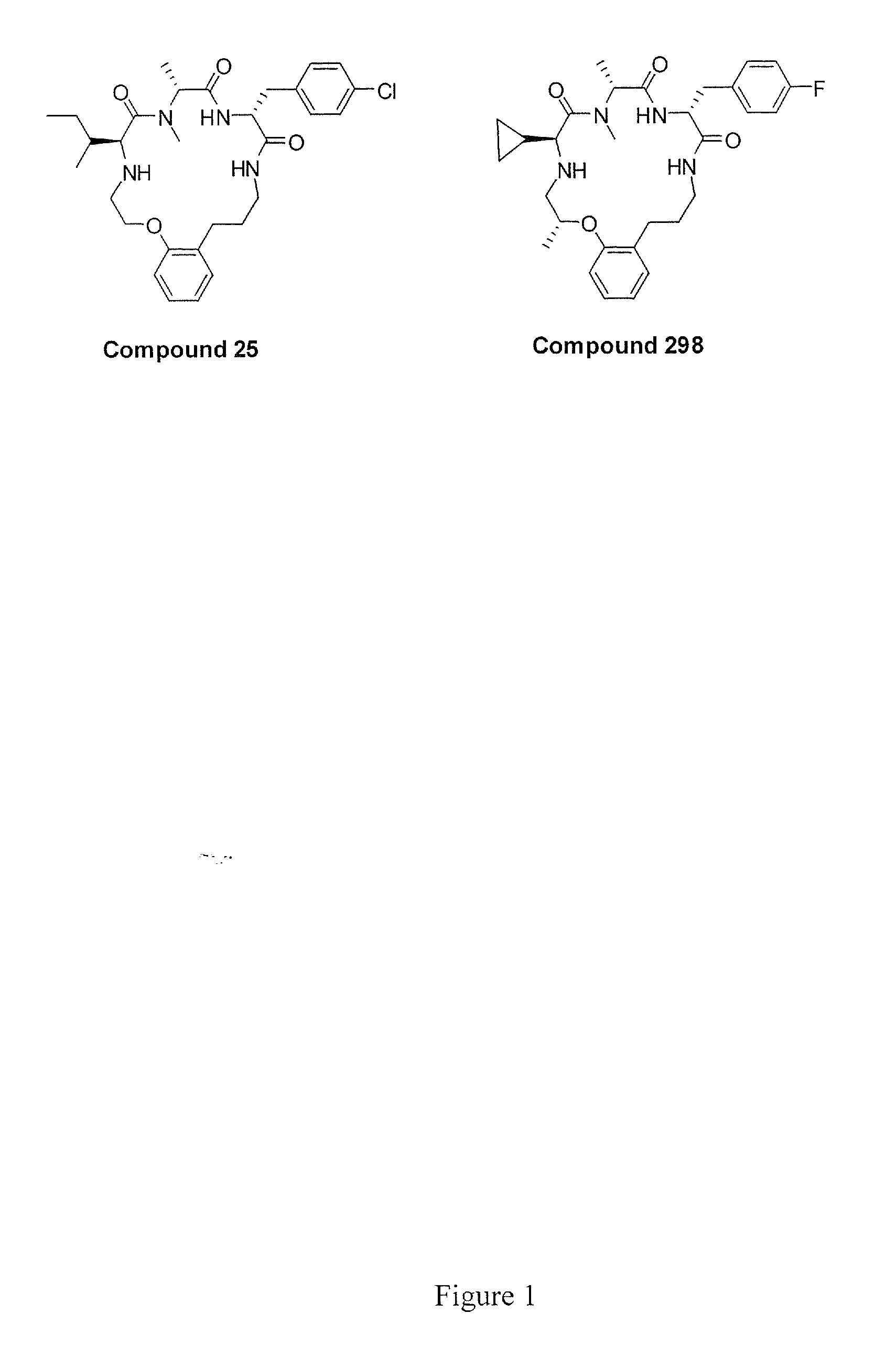
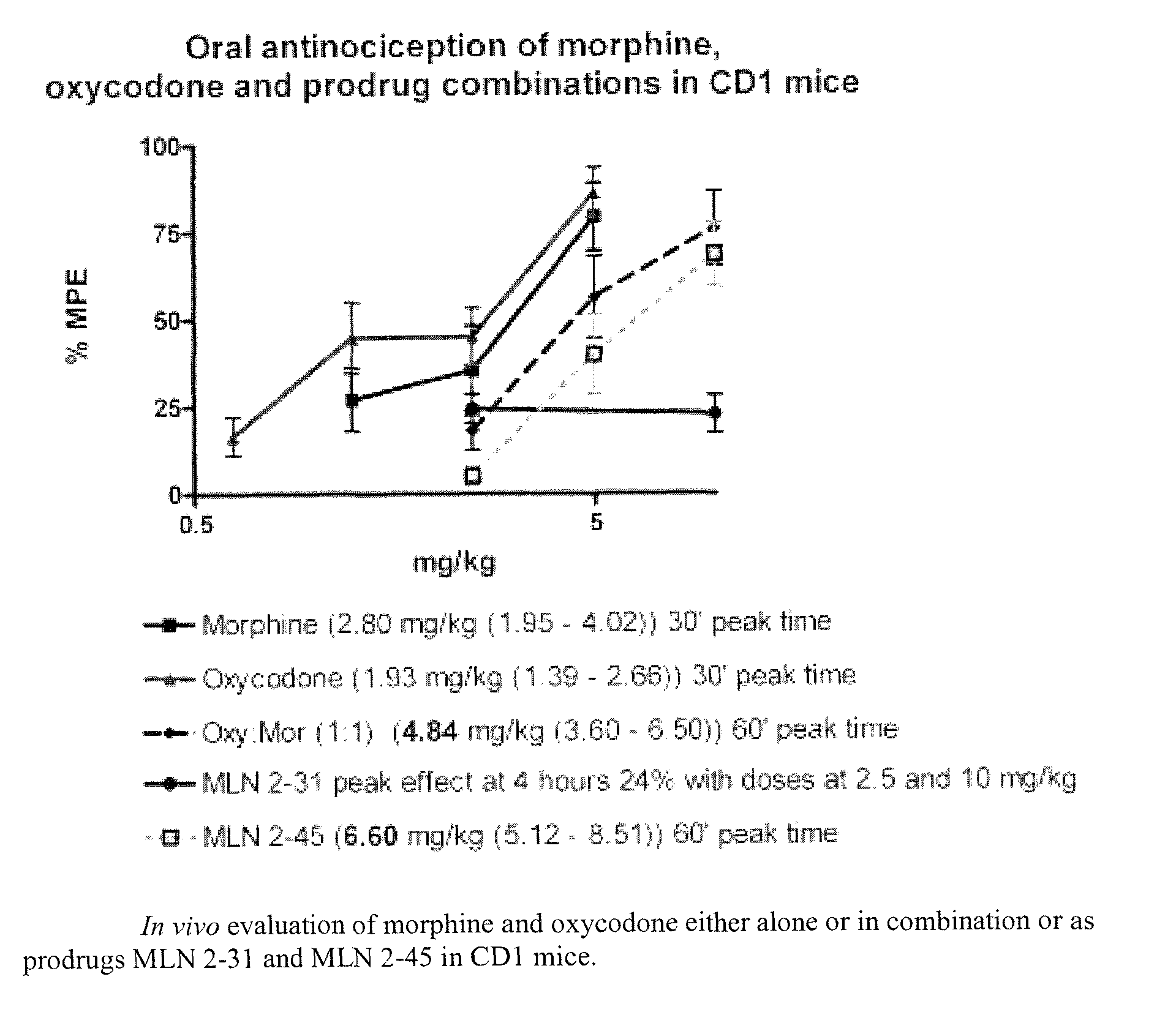
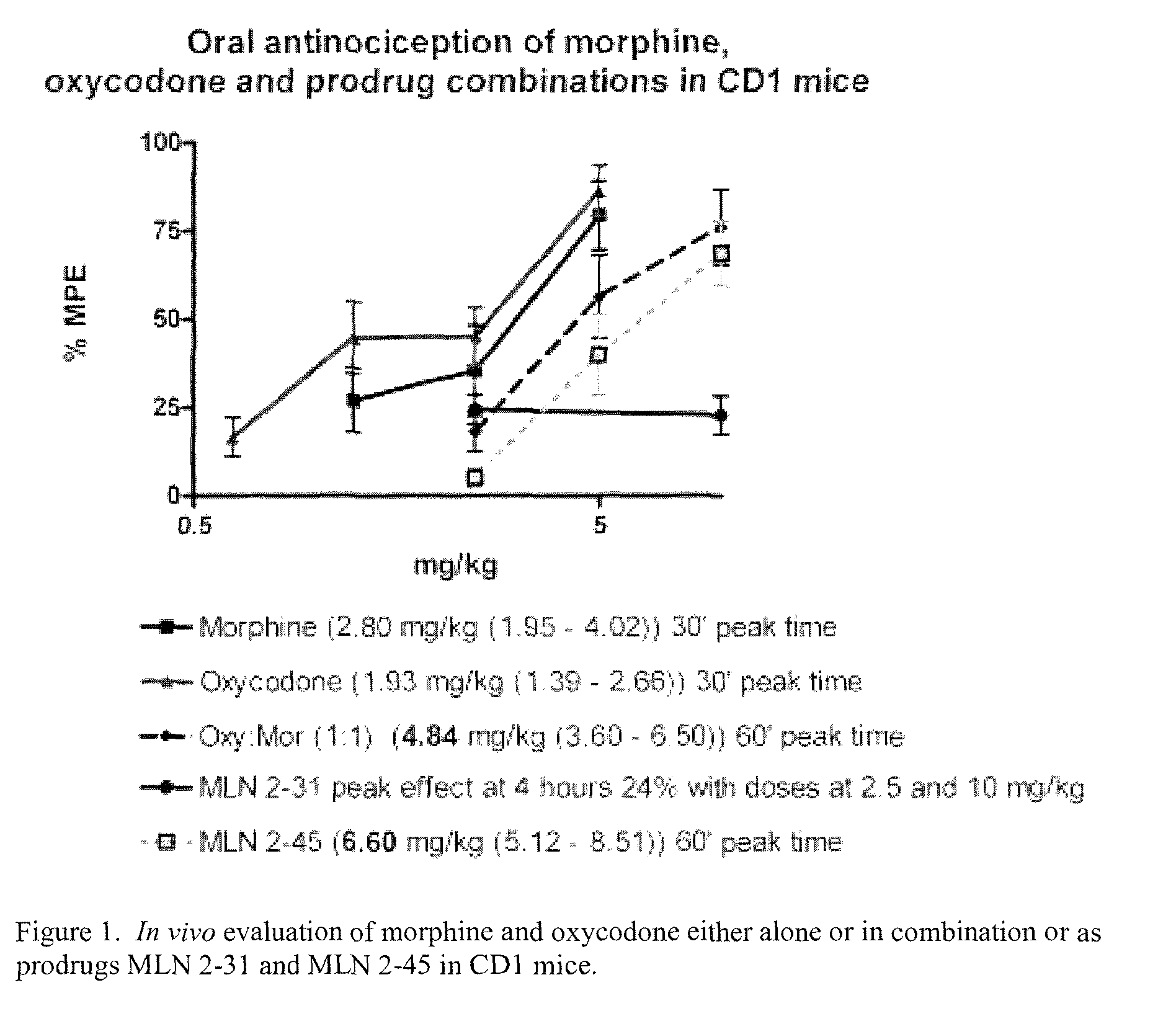
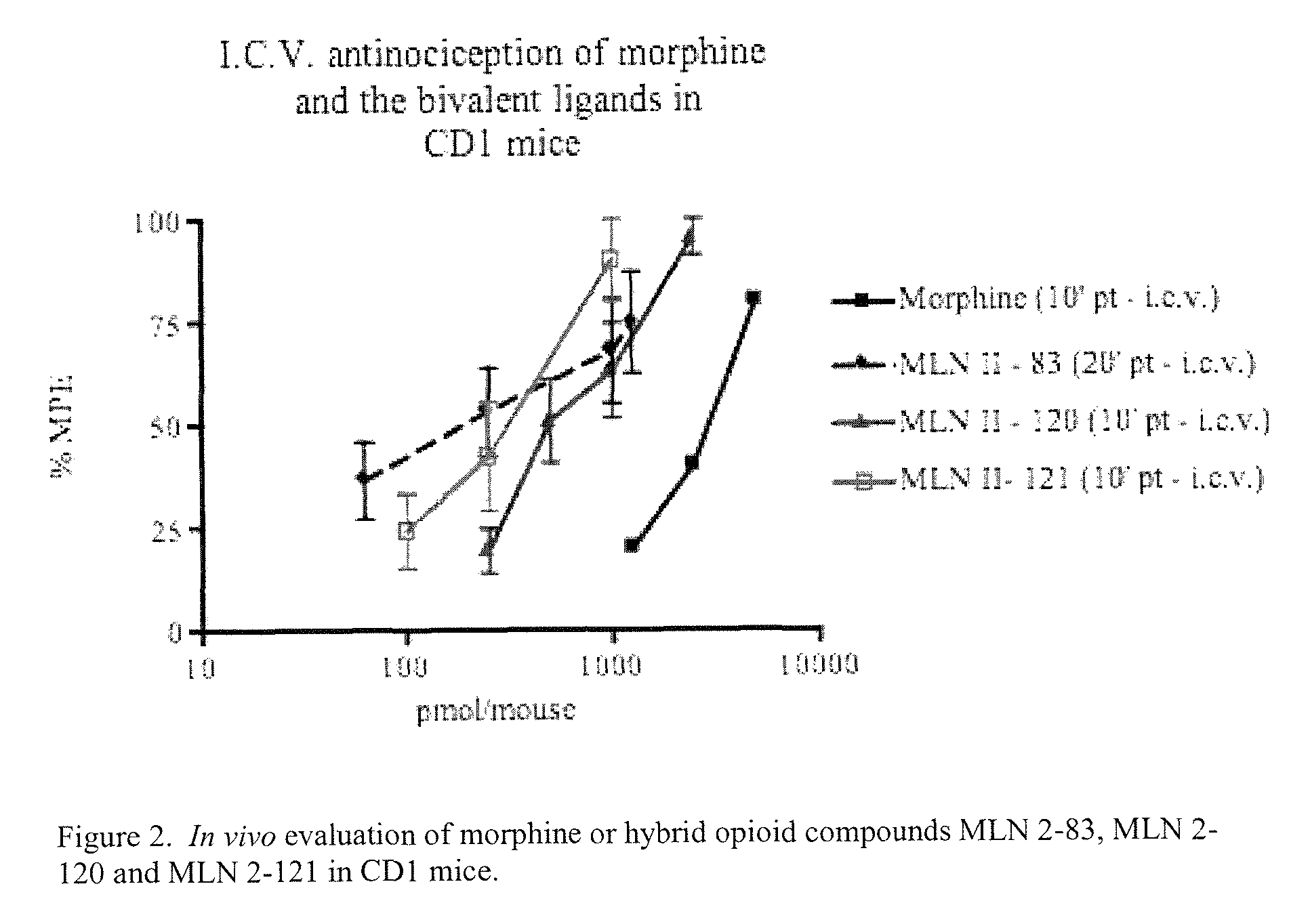
Hybrid Opioid Compounds and Compositions
Disclosed are hybrid opioid compounds, mixed opioid salts, compositions comprising the hybrid opioid compounds and mixed opioid salts, and methods of use thereof. More particularly, in one aspect the hybrid opioid compound includes at least two opioid compounds that are covalently bonded to a linker moiety. In another aspect, the hybrid opioid compound relates to mixed opioid salts comprising at least two different opioid compounds or an opioid compound and a different active agent. Also disclosed are pharmaceutical compositions, as well as to methods of treating pain in humans using the hybrid compounds and mixed opioid salts.
Owner:QRXPHARMA
Diversion-resistant opioid formulations
The present invention provides a composition comprising an opioid agonist, and a polymer-antagonist conjugate. The polymer-antagonist conjugate preferably does not hydrolyze upon administration to a patient, and does not bind to the opioid receptors. The covalent bond between the polymer and the antagonist in the conjugate is broken over a defined period of time to release the antagonist into the formulation. The released antagonist attenuates the liking of the agonist, thereby eliminating the incentive to the diversion of the medicines.
Owner:ELYSIUM THERAPEUTICS
Robust sustained release formulations of oxymorphone
InactiveUS20080085305A1Avoid dose dumpingHigh drug safetyPowder deliveryOrganic chemistryOxymorphoneSustained Release Formulations
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Method of treatment and/or prophylaxis
InactiveUS20030199424A1Easy to prepareHigh viscosityHeavy metal active ingredientsBiocideVertebrate AnimalsPsychiatry
The present invention is directed to the use of angiotensin II receptor I (AT1 receptor) antagonists for the treatment, prophylaxis, reversal and / or symptomatic relief of a neuropathic condition, especially a peripheral neuropathic condition such as painful diabetic neuropathy, in vertebrate animals and particularly in human subjects. The present invention also discloses the use of AT1 receptor antagonists for preventing, attenuating or reversing the development of reduced opioid sensitivity, and more particularly reduced opioid analgesic sensitivity, in individuals and especially in individuals having, or at risk of developing, a neuropathic condition.
Owner:QUEENSLAND UNIV OF
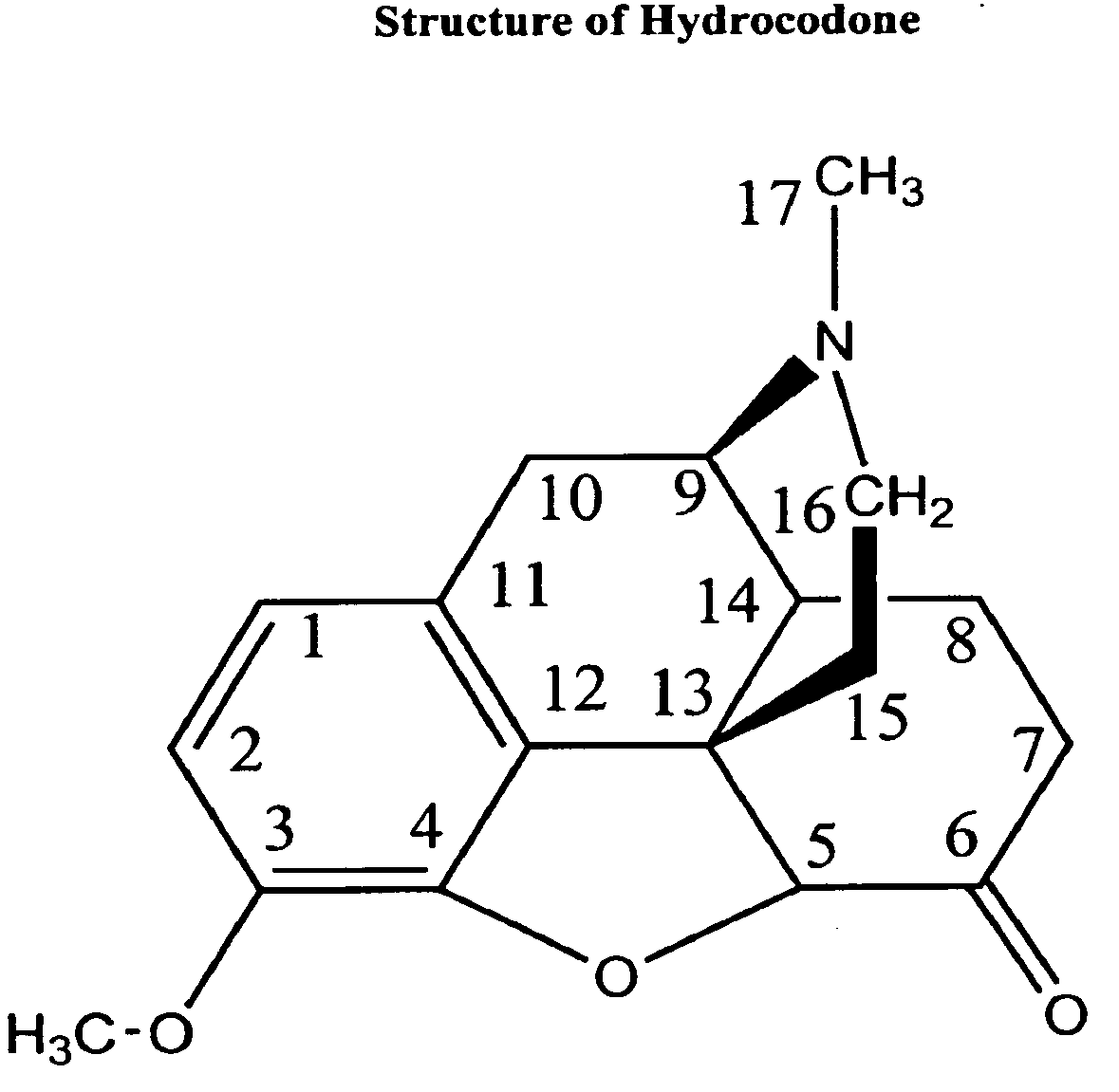
Formulations of nonopioid and confined opioid analgesics
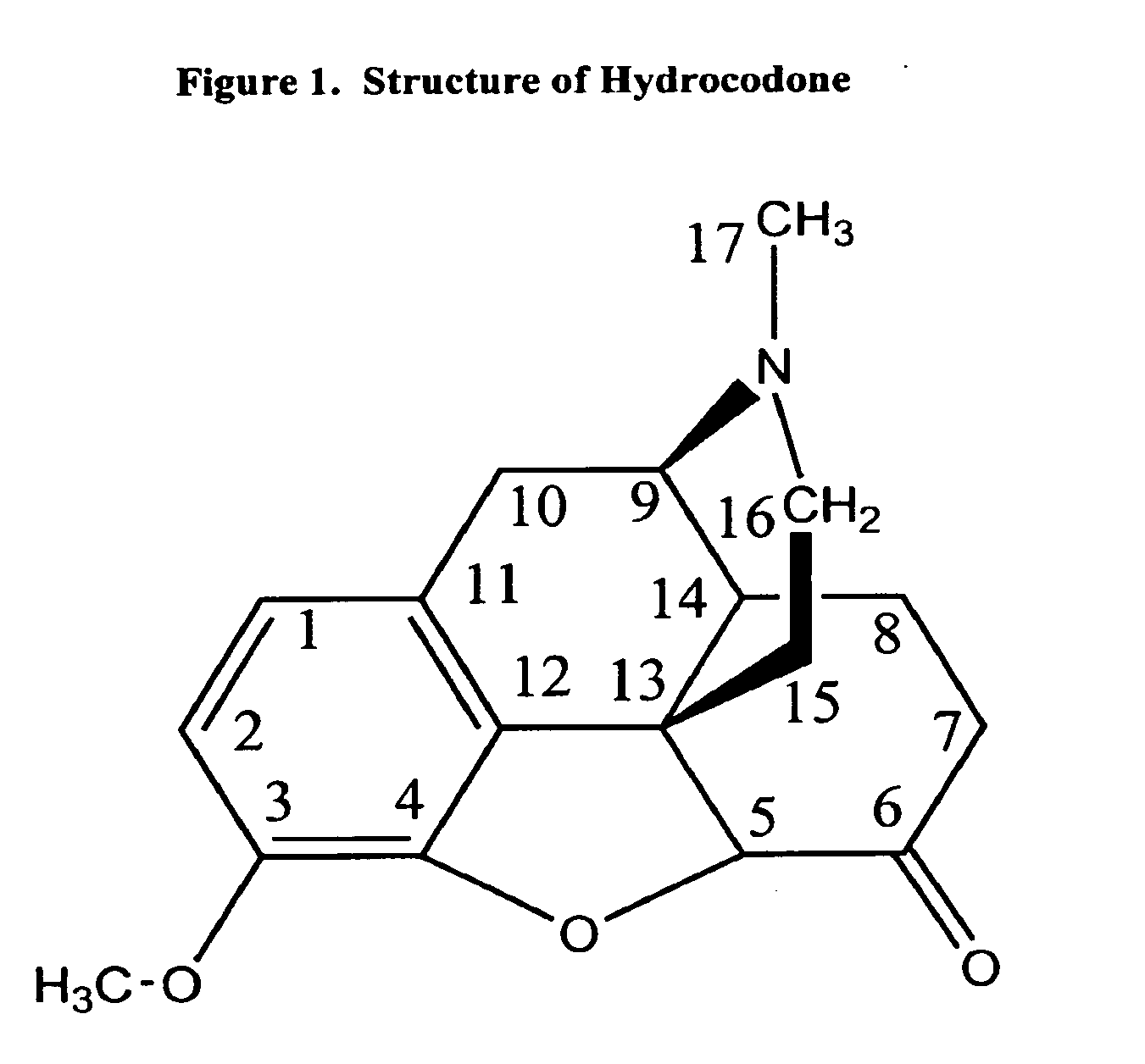
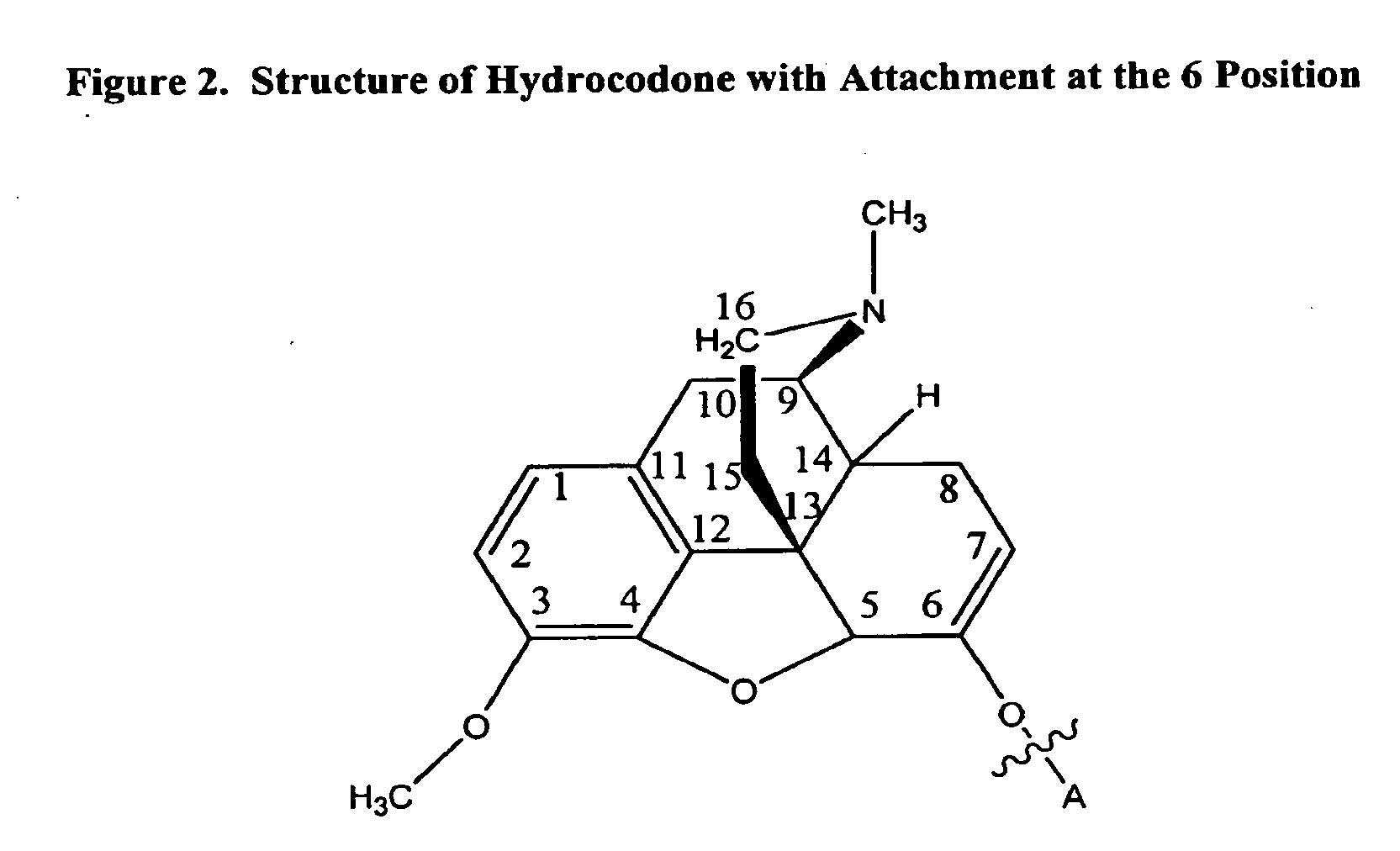
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Abuse-resistant oral dosage forms and method of use thereof
InactiveUS20080166405A1Antagonizing the opioid effect of an opioid agonistBiocidePowder deliveryOpioid AgonistOpioid antagonist
An opioid-antagonist oral dosage form which does not release a therapeutically effective amount of the opioid antagonist when the oral dosage form is orally administered to a human being, but whereby a physical alteration of the oral dosage form results in a release of the therapeutically effective amount of the opioid antagonist. An embodiment of the oral dosage form includes an opioid-antagonist layer coated onto a biologically inert pellet, and a non-releasing membrane coated onto the opioid-antagonist layer. Optionally, the oral dosage form can also include an opioid agonist, such that a method of preventing the abuse of an oral dosage form of an opioid agonist is provided by forming the oral dosage form including an opioid agonist and an opioid antagonist.
Owner:MEHTA ATUL M
Methods of Reducing Side Effects of Analgesics
InactiveUS20100227876A1Decreased gastrointestinal motilityReduce resistanceBiocideAnimal repellantsMu-Opioid Receptor AgonistsSide effect
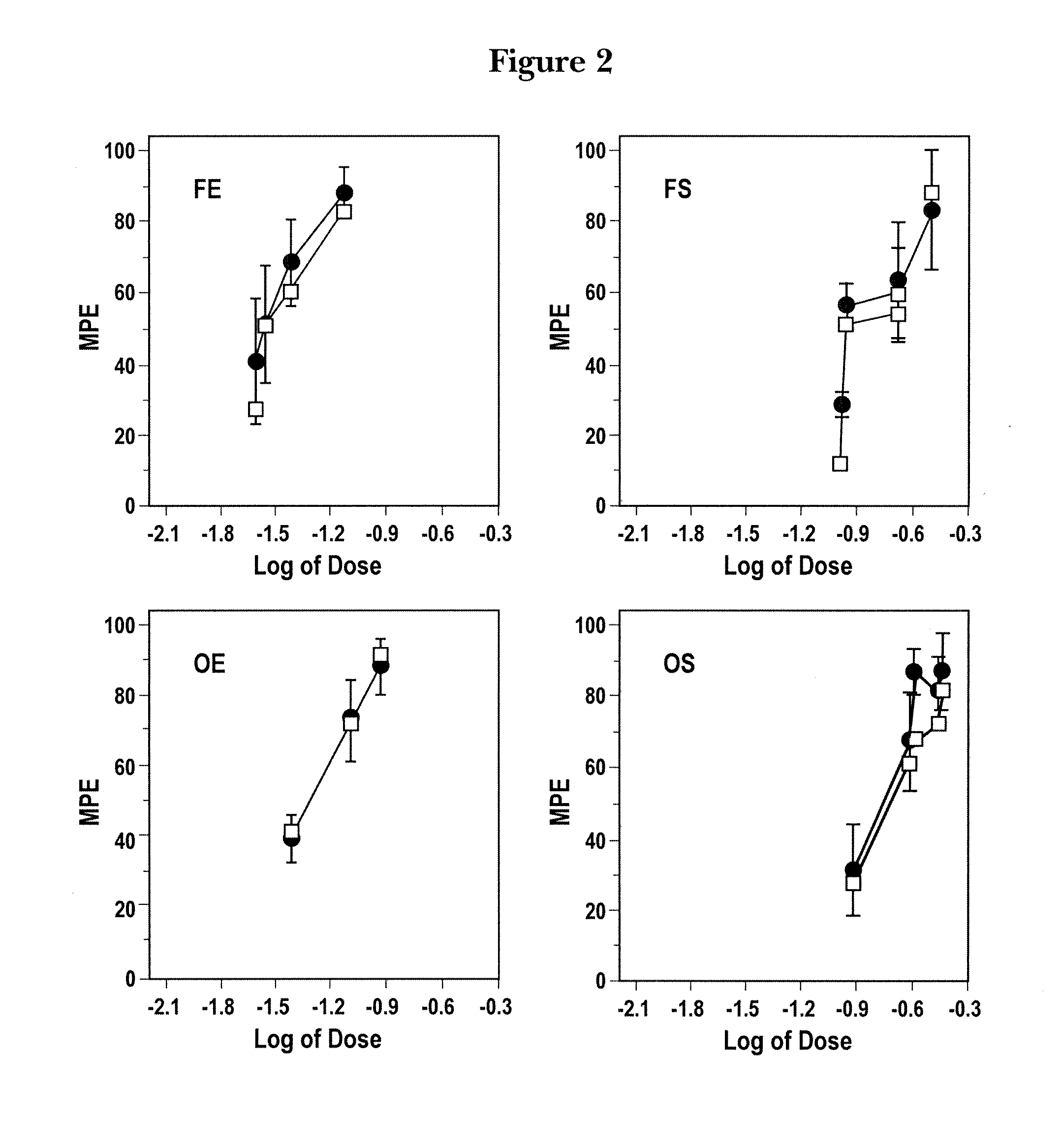
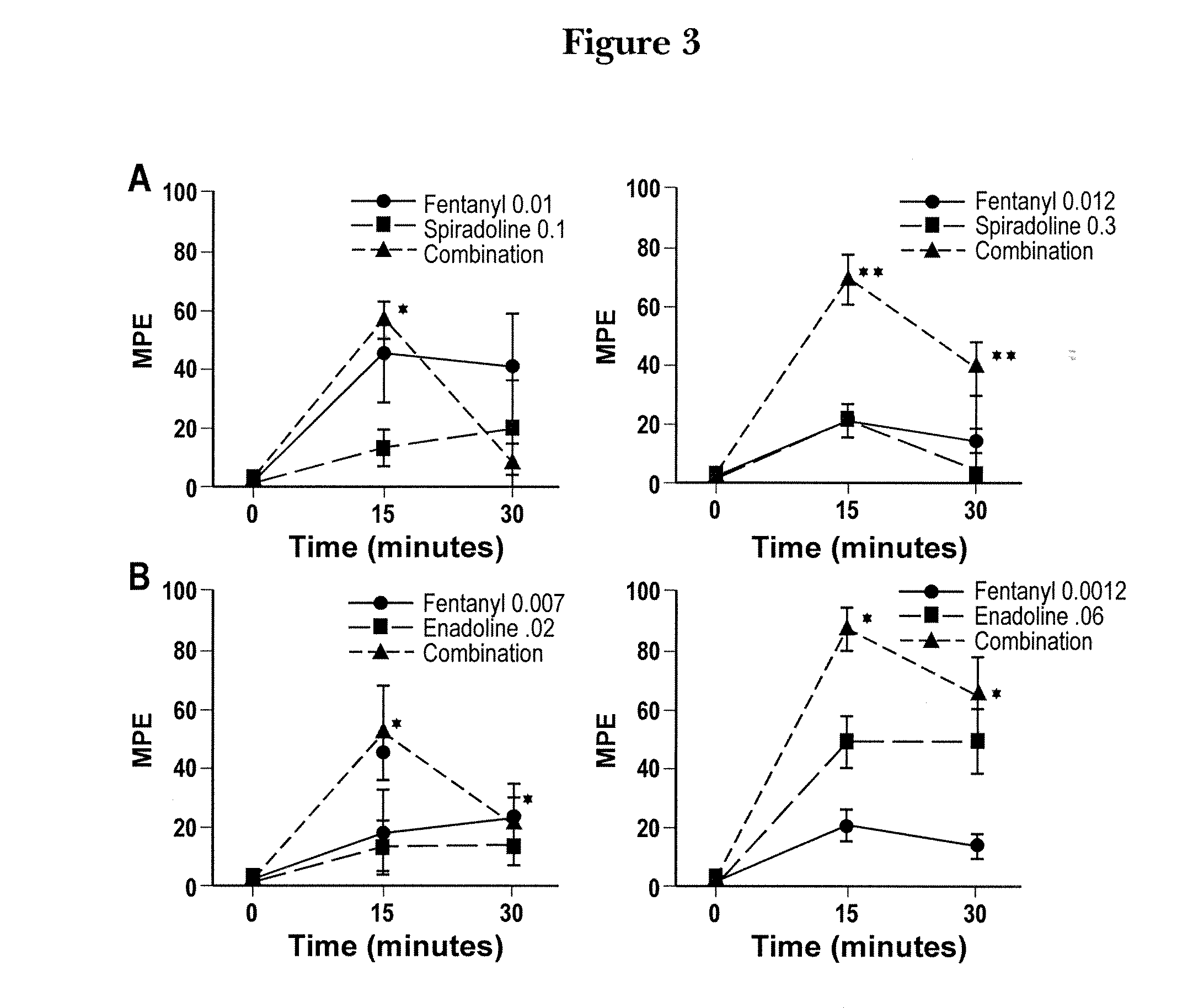
The invention provides for compositions and methods of reducing pain in a subject by administering a combination of mu-opioid receptor agonist, kappa1-opioid receptor agonist and a nonselective opioid receptor antagonist in amounts effective to reduce pain and ameliorate an adverse side effect of treatment combining opioid-receptor agonists. The invention also provides for methods of enhancing an analgesic effect of treatment with an opioid-receptor agonist in a subject suffering from pain while reducing an adverse side effect of the treatment. The invention also provides for methods of reducing the hyperalgesic effect of treatment with an opioid-receptor agonist in a subject suffering from pain while reducing an adverse side effect of the treatment. The invention further provides for methods of promoting the additive analgesia of pain treatment with an opioid-receptor agonist in a subject in need while reducing an adverse side effect of the treatment.
Owner:RECHFENSEN
Methods of using macrocyclic agonists of the ghrelin receptor for treatment of gastrointestinal motility disorders
The present invention provides novel conformationally-defined macrocyclic compounds that have been demonstrated to be selective agonists of the ghrelin receptor (growth hormone secretagogue receptor, GHS-R1a and subtypes, isoforms and variants thereof). Such compounds are useful as medicaments for treatment and prevention of a range of medical conditions characterized by disturbed gastrointestinal motility including, but not limited to, post-surgical gastroparesis and post-operative ileus in combination with opioid-induced bowel dysfunction. These agents are effective for multiple disorders at dose levels equivalent to those required to treat a single disorder.
Owner:OCERA THERAPEUTICS INC
Particles containing an opioid receptor antagonist and methods of use
InactiveUS20110250278A1Good effectPromote absorptionBiocidePowder deliverySide effectNK1 receptor antagonist
Particles comprising an opioid receptor antagonist as well as methods of their use and methods of their preparation are provided herein. Such particles may be used for treating and preventing opioid-induced side effects in patients, and may be provided to chronic opioid users as well.
Owner:UNIVERSITY OF CHICAGO
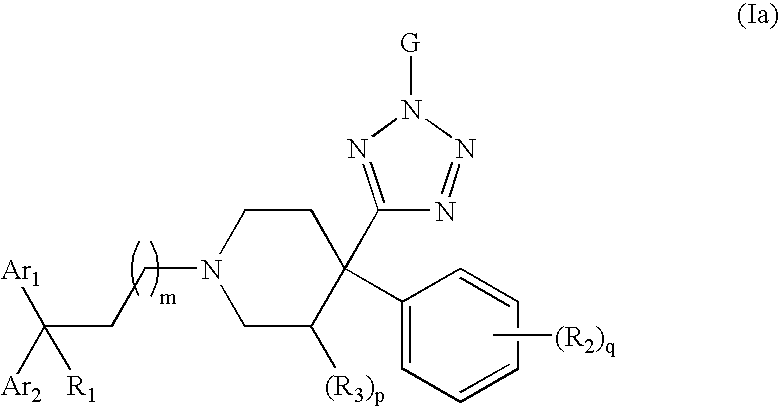
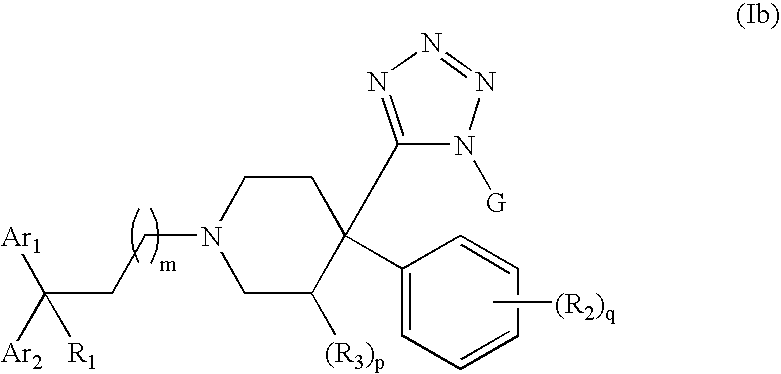
Therapeutic agents useful for treating pain
4-Tetrazolyl-4-phenylpiperidine Compounds, compositions comprising an effective amount of a 4-Tetrazolyl-4-phenylpiperidine Compound, methods for treating or preventing pain or diarrhea in an animal comprising administering to an animal in need thereof an effective amount of a 4-Tetrazolyl-4-phenylpiperidine Compound and methods for stimulating opioid-receptor function in a cell comprising contacting a cell capable of expressing an opioid receptor with an effective amount of a 4-Tetrazolyl-4-phenylpiperidine Compound are disclosed.
Owner:PURDUE PHARMA LP
Compositions and methods for enhancing analgesic potency of covalently bound-compounds, attenuating its adverse side effects, and preventing their abuse
InactiveUS20100144645A1Lower potentialAmenable to synthesizing conjugatesBiocideNervous disorderChemical MoietyOpioid antagonist
The invention generally relates to compositions and methods with covalently bound compounds, such as controlled substances covalently attached to a chemical moiety, and opioid antagonists or covalently bound opioid antagonists to enhance analgesic potency and / or attenuate one or more adverse effects of covalently bound compounds, including adverse side effect(s) in humans such as nausea, vomiting, dizziness, headache, sedation (somnolence), physical dependence or pruritis. This invention relates to compositions and methods for selectively enhancing the analgesic potency of a covalently bound compound and simultaneously attenuating anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects associated with the administration of a covalently bound compound. The methods of the invention comprise administering to a subject an analgesic or sub-analgesic amount of a covalently bound compound and an amount of excitatory opioid receptor antagonist such as naltrexone or nalmefene effective to enhance the analgesic potency of a covalently bound compound and attenuate the anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects of covalently bound compound. The invention also relates to the addition of covalently-bound opioid antagonists to the compositions containing covalently bound compounds such that if the compositions are subjected to manipulation by illicit chemists, the opioid antagonist is released effectively reducing or eliminating the euphoric effect of the covalently bound compounds.
Owner:SHIRE PLC
Compositions Comprising Enzyme-Cleavable Phenol-Modified Opioid Prodrugs and Inhibitors Thereof
InactiveUS20110262360A1Eliminate side effectsPatient compliance is goodBiocideNervous disorderControlled releasePhenol
Pharmaceutical compositions and their methods of use are provided, where the pharmaceutical compositions comprise a phenol-modified opioid prodrug that provides enzymatically-controlled release of a phenolic opioid, and an enzyme inhibitor that interacts with the enzyme(s) that mediates the enzymatically-controlled release of the phenolic opioid from the phenol-modified opioid prodrug so as to modify enzymatic cleavage of the phenol-modified opioid prodrug.
Owner:SIGNATURE THERAPEUTICS
Hybrid opioid compounds and compositions
Disclosed are hybrid opioid compounds, mixed opioid salts, compositions comprising the hybrid opioid compounds and mixed opioid salts, and methods of use thereof. More particularly, in one aspect the hybrid opioid compound includes at least two opioid compounds that are covalently bonded to a linker moiety. In another aspect, the hybrid opioid compound relates to mixed opioid salts comprising at least two different opioid compounds or an opioid compound and a different active agent. Also disclosed are pharmaceutical compositions, as well as to methods of treating pain in humans using the hybrid compounds and mixed opioid salts.
Owner:QRXPHARMA
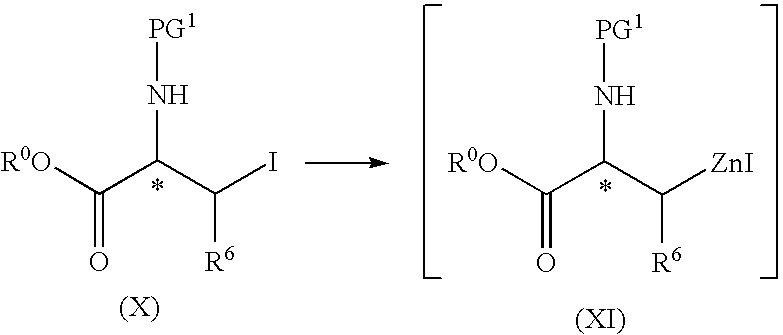
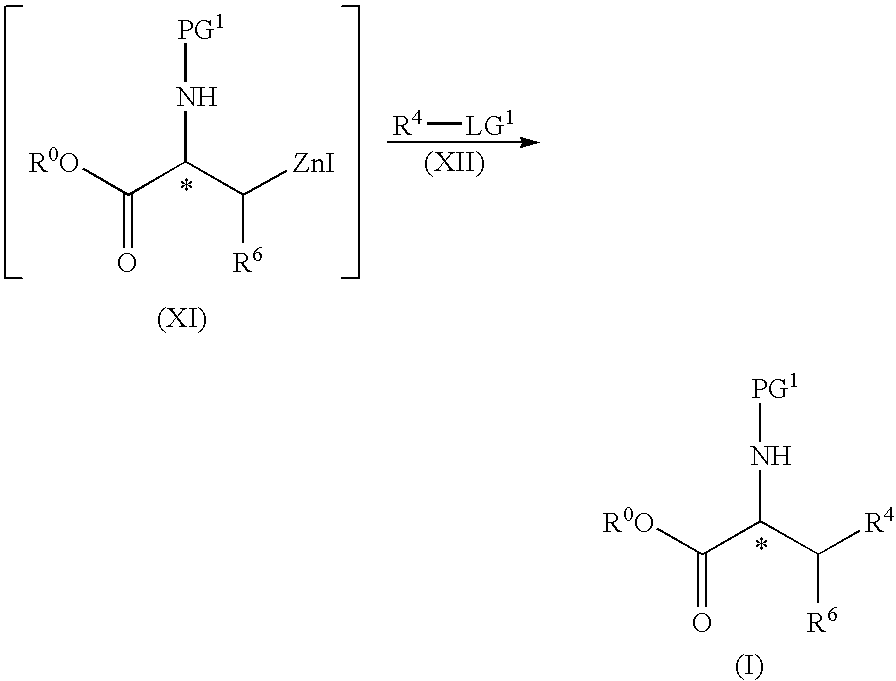
Process for the preparation of protected L-alanine derivatives
ActiveUS8710256B2Carbamic acid derivatives preparationOrganic compound preparationMedicinal chemistryL-alanosine
Owner:JANSSEN PHARMA NV
Composition Comprising a Therapeutic Agent and a Respiratory Stimulant and Methods for the Use Thereof
The present disclosure provides a safe method for anesthesia or the treatment of pain by safely administering an amount of active agent to a patient while reducing the incidence or severity of suppressed respiration. The present disclosure provides a pharmaceutical composition comprising a therapeutic agent and a chemoreceptor respiratory stimulant. In one aspect, the compositions oppose effects of respiratory suppressants by combining a chemoreceptor respiratory stimulant with an opioid receptor agonist or other respiratory-depressing drug. The combination of the two chemical agents, that is, the therapeutic agent and the respiratory stimulant, may be herein described as the “drugs.” The present compositions may be used to treat acute and chronic pain, sleep apnea, and other conditions, leaving only non-lethal side effects.
Owner:HSU JOHN
Opioid salts and formulations exhibiting anti-abuse and anti-dose dumping properties
Owner:PISGAH LAB
Sublingual buprenorphine spray
ActiveUS9216175B2Discourages improper administrationOrganic active ingredientsNervous disorderSublingual buprenorphinePharmacology
The invention provides sublingual formulations containing buprenorphine, a pharmaceutically acceptable salt thereof, or a derivative thereof. The invention further provides sublingual formulations containing buprenorphine and naloxone, pharmaceutically acceptable salts thereof or derivatives thereof. The invention further provides a method of treating pain or opioid dependence by administering sublingual formulations containing buprenorphine, a pharmaceutically acceptable salt thereof, or a derivative thereof to a patient in need thereof.
Owner:BENUVIA OPERATIONS LLC
Amino acid and peptide prodrugs of opioid analgesics with reduced gi side-effects
InactiveUS20090192095A1Minimizing gastrointestinal side effectEliminate the effects ofBiocideNervous disorderSide effectCarbamate
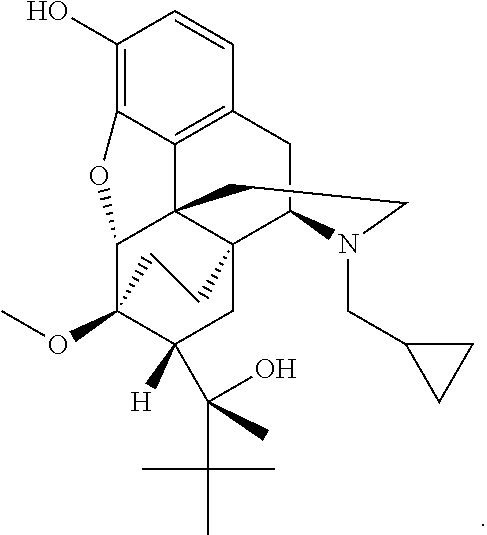
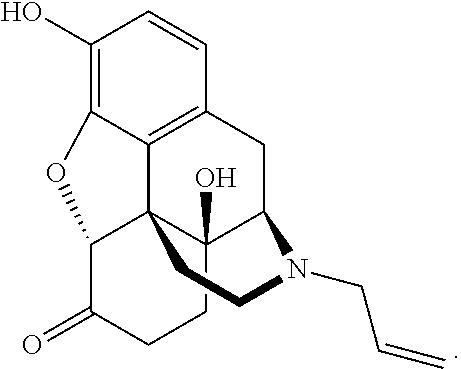
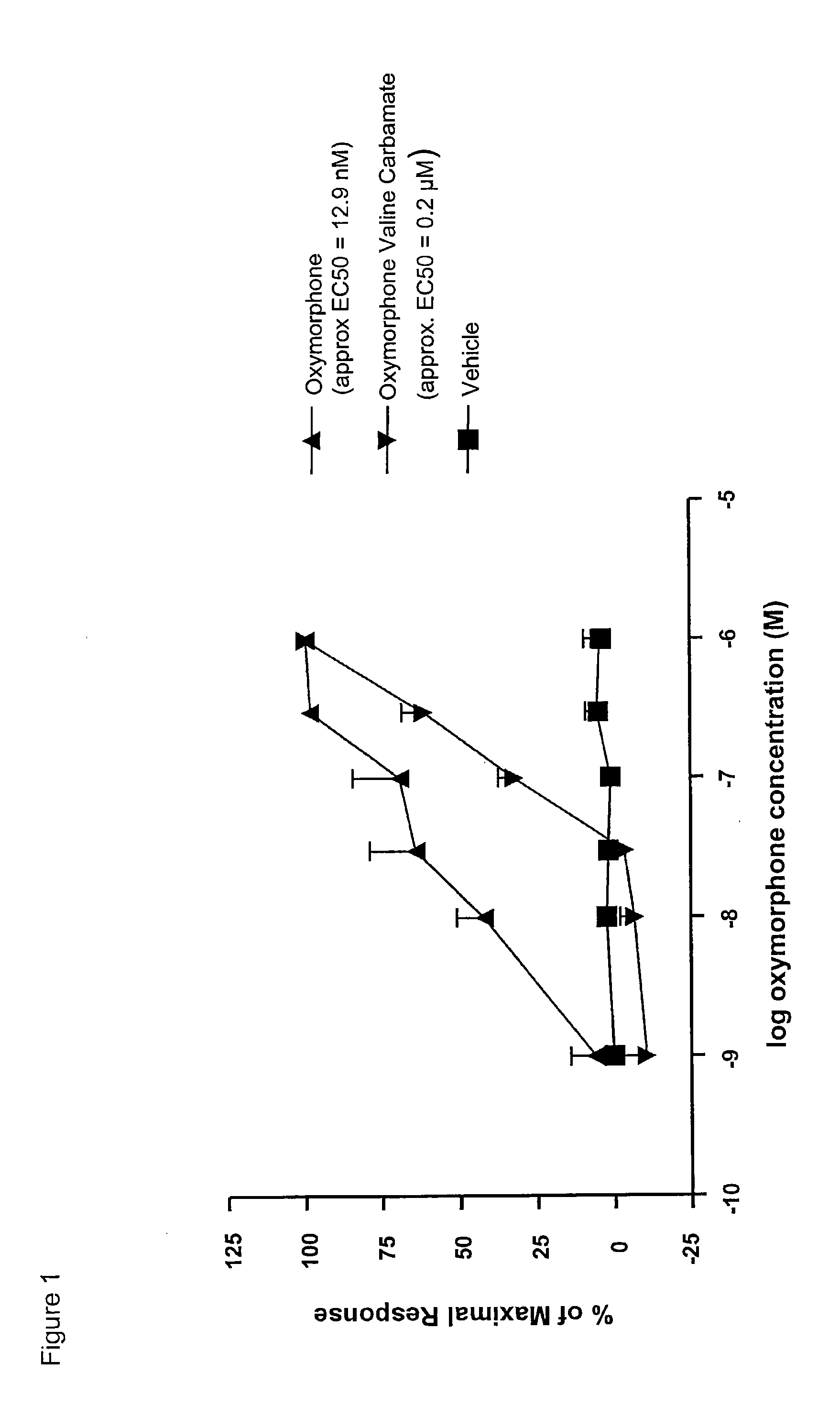
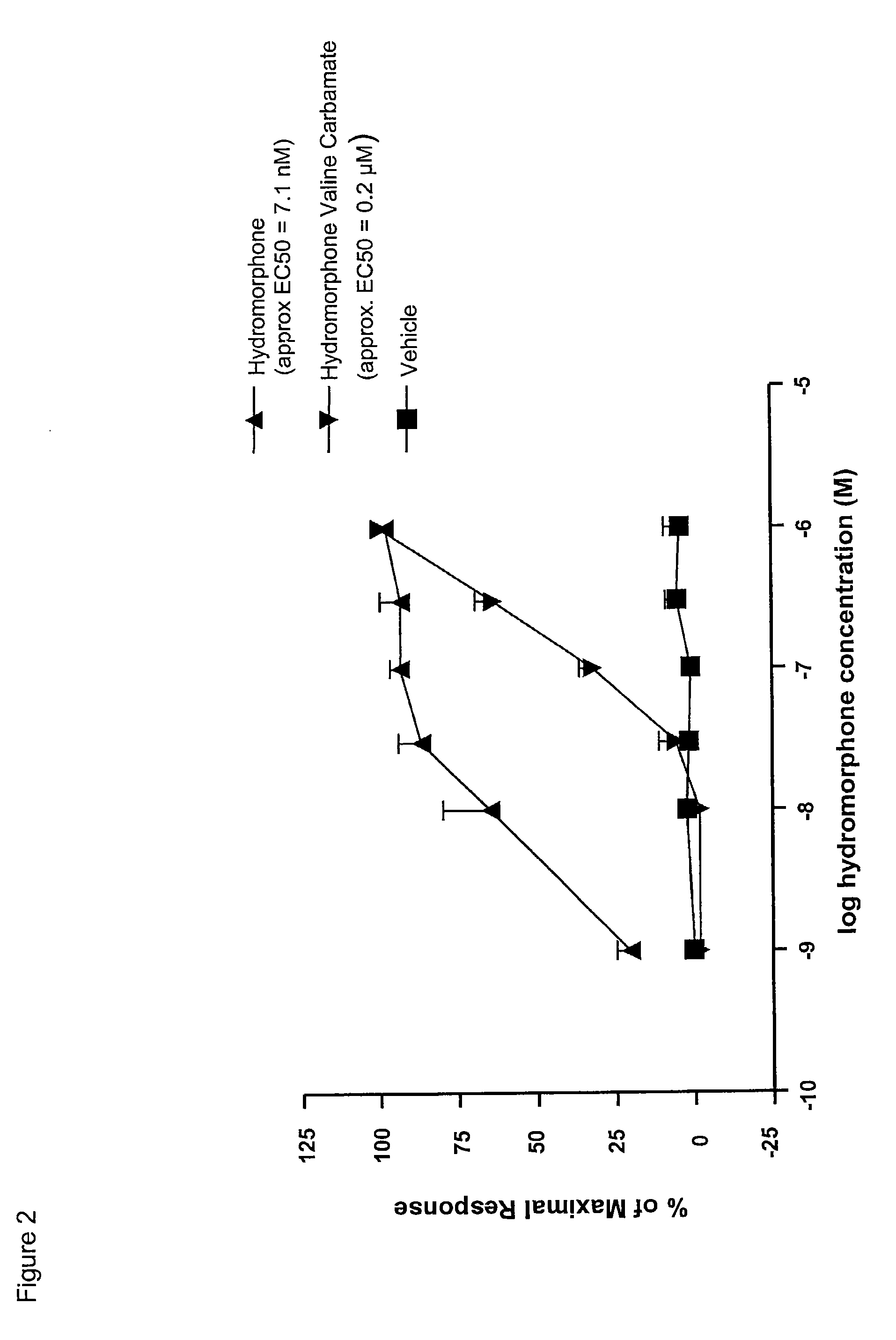
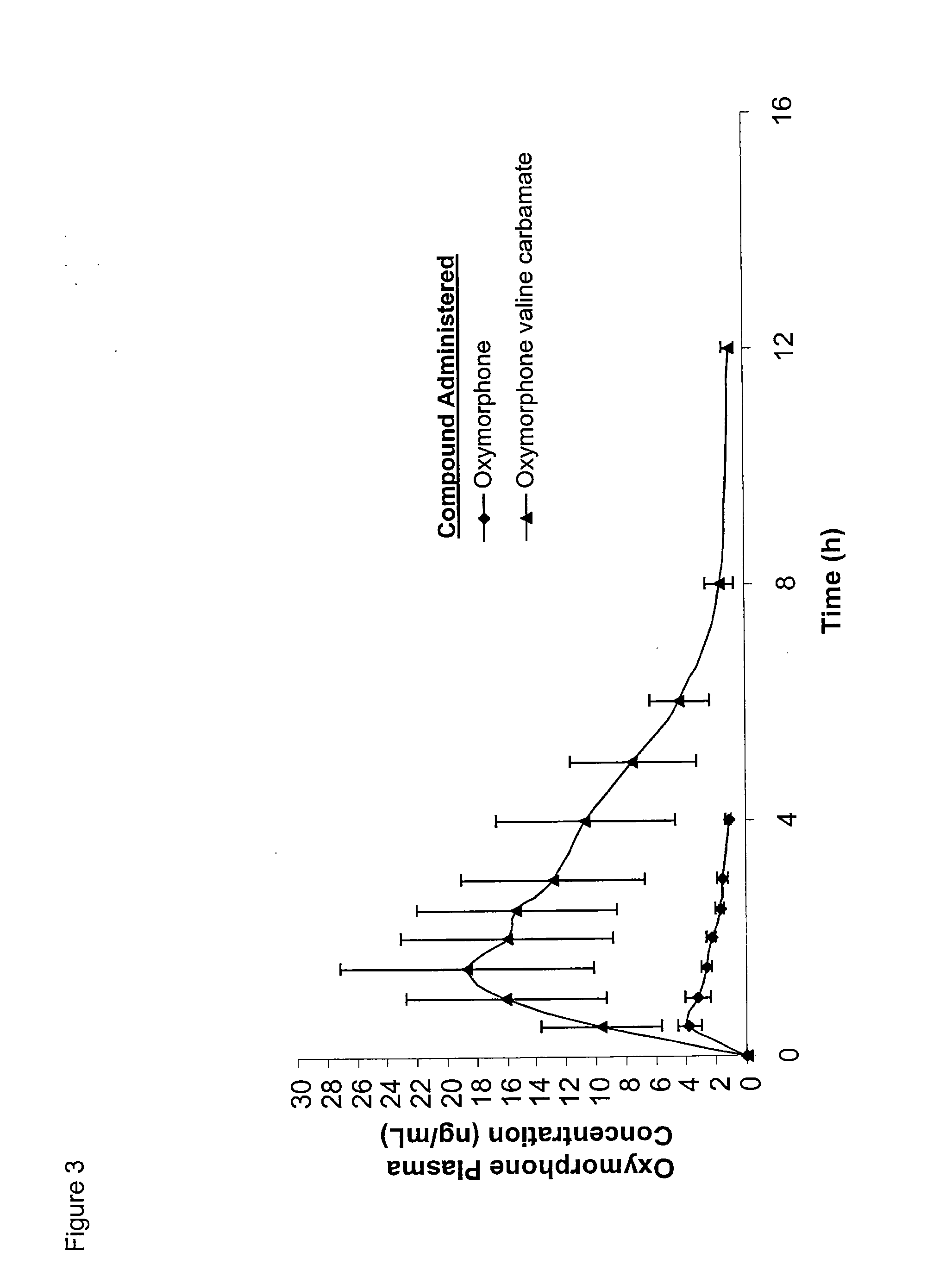
The present invention relates to methods for reducing gastrointestinal side effects in a subject, the gastrointestinal side effects being associated with the administration of an opioid analgesic. The methods comprise orally administering an opioid prodrug or pharmaceutically acceptable salt thereof to a subject, wherein the opioid prodrug is comprised of an opioid analgesic covalently bonded through a carbamate linkage to a peptide of 1-5 amino acids in length, and wherein upon oral administration, the prodrug or pharmaceutically acceptable salt minimizes at least one gastrointestinal side effect associated with oral administration of the opioid analgesic alone. Compositions for use with the method are also provided.
Owner:SHIRE PLC
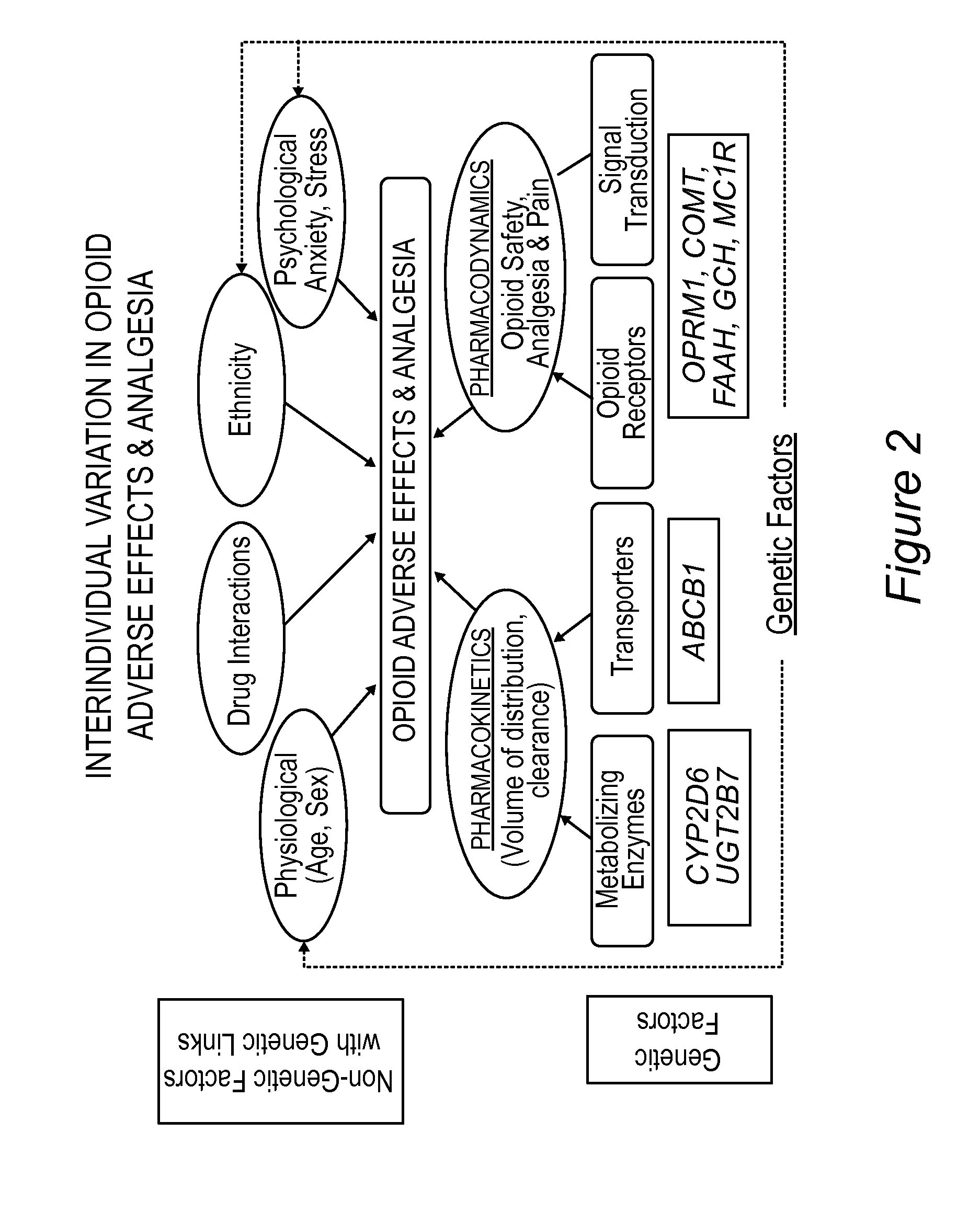
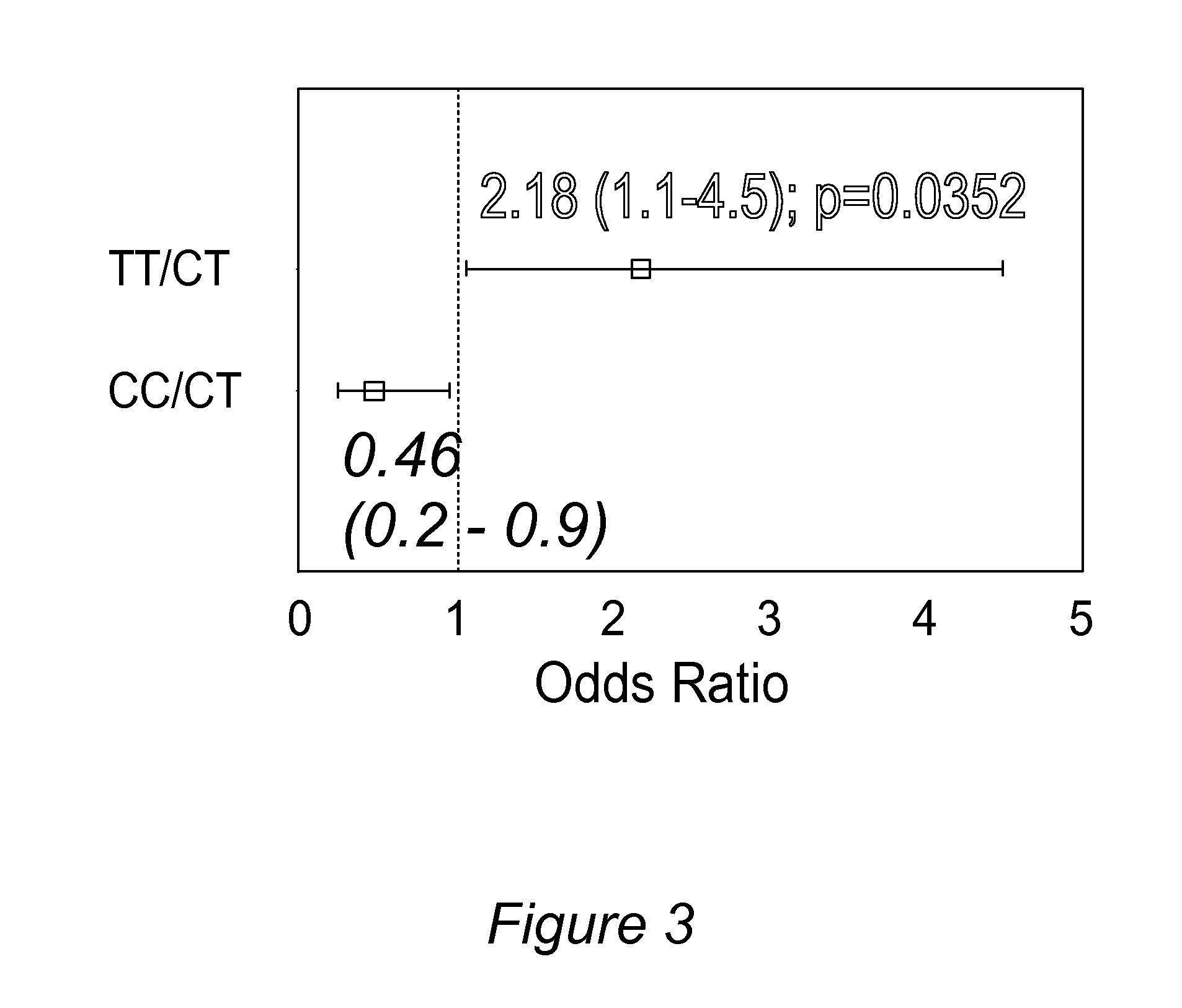
Personalized pain management and anesthesia: preemptive risk identification and therapeutic decision support
ActiveUS20140371256A1Maximize pain reliefAdverse effect in subjectBiocideMicrobiological testing/measurementPersonalizationSide effect
Methods and compositions disclosed herein generally relate to methods of improving clinical and economic outcomes to address adverse effects related to anesthesia, analgesics, opioids, and inadequate pain relief. Embodiments of the invention relate to the association between genes, specific polymorphisms of genes, and non-genetic factors with inadequate pain relief and anesthesia-, analgesic, and / or opioid-related adverse effects. Embodiments of the invention can be used to determine and manage patient risk factors for development of adverse perioperative effects and can allow for personalized anesthesia and pain management for improvement of pain control and reduction of anesthesia-, analgesic-, and opioid-related adverse outcomes. These methods and compositions apply to non-surgical pain management with opioids. Therefore, patients who are genetically predisposed to risk of inadequate pain relief and / or serious side effects from anesthesia, analgesics, and / or opioids can be identified and individualized treatment plans developed for implementation by the clinician to improve clinical and economic outcomes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Buprenorphine analogs
ActiveUS8969358B2Eliminate the effects ofReduce and prevent constipationBiocideNervous disorderStereoisomerismConstipation
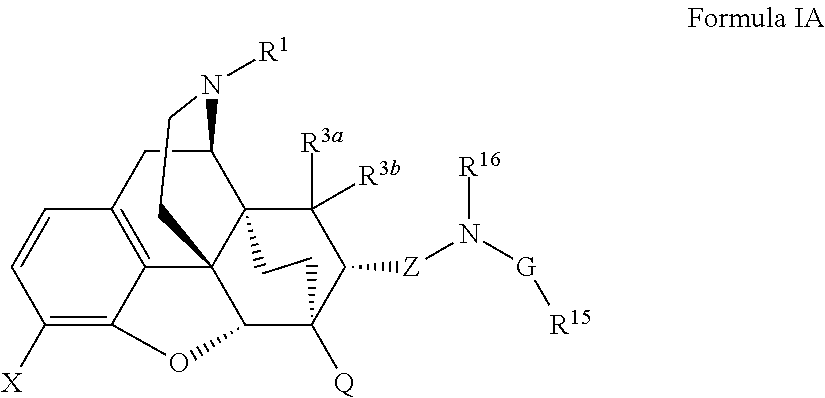
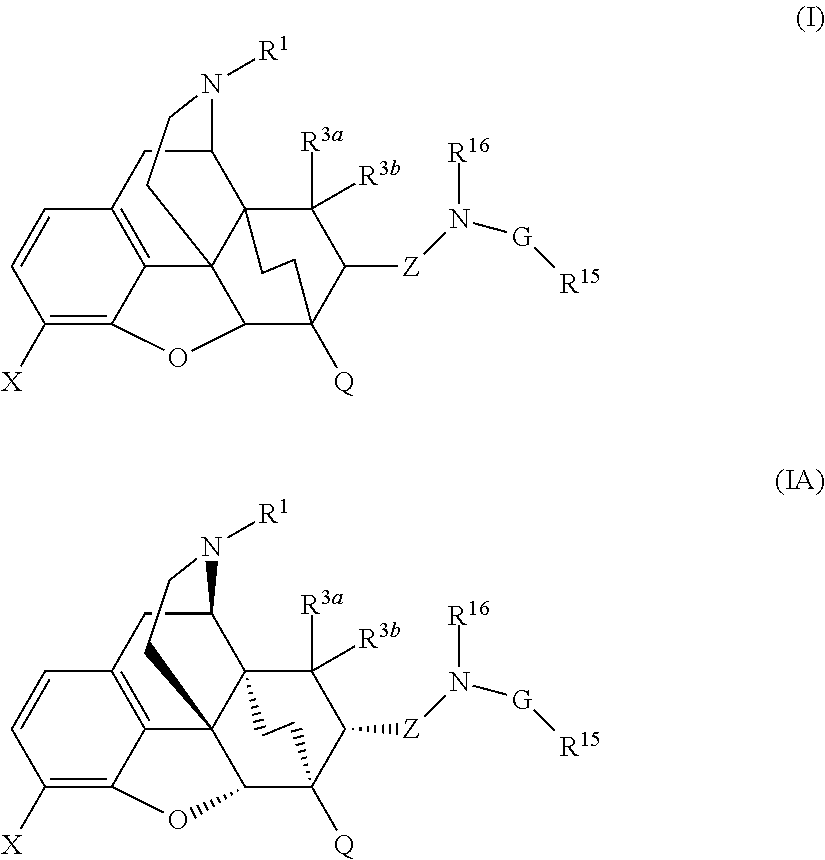
The present invention is directed to Buprenorphine Analog compounds of Formula I, II, III, IV or V and including various stereoisomers (such as Formula IA shown below), wherein R1, R3a, R3b, R16, R15, G, Q, X, A and Z are as defined herein.Compounds of the Invention may be useful for preparing medicaments useful for treating pain, constipation, and other conditions modulated by activity of opioid and ORL-1 receptors. Compounds of the Invention may be useful for treating Conditions such as pain, constipation, and others modulated by activity of opioid and ORL-1 receptors.
Owner:PURDUE PHARMA LP
Methods and compositions to prevent addiction
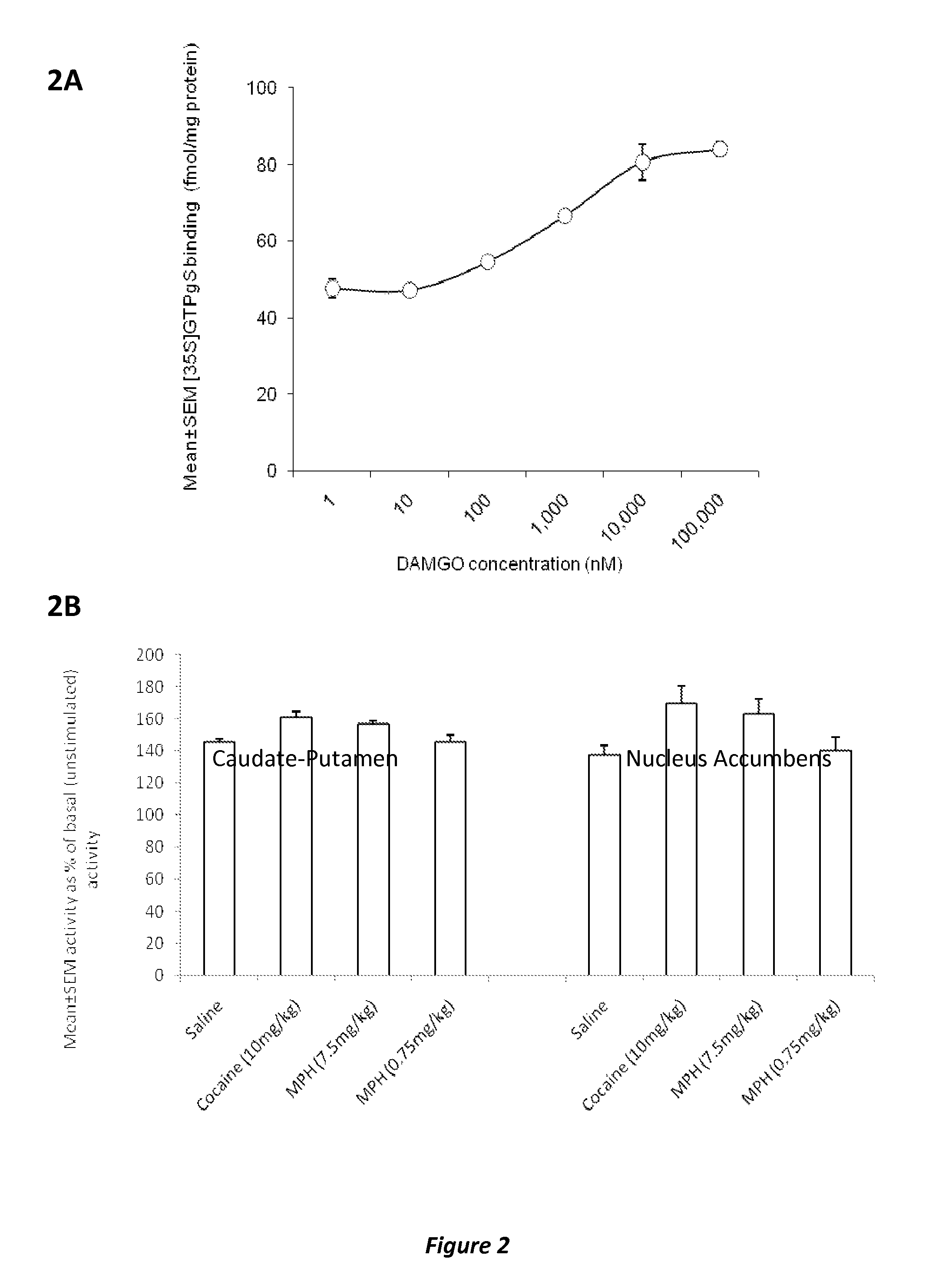
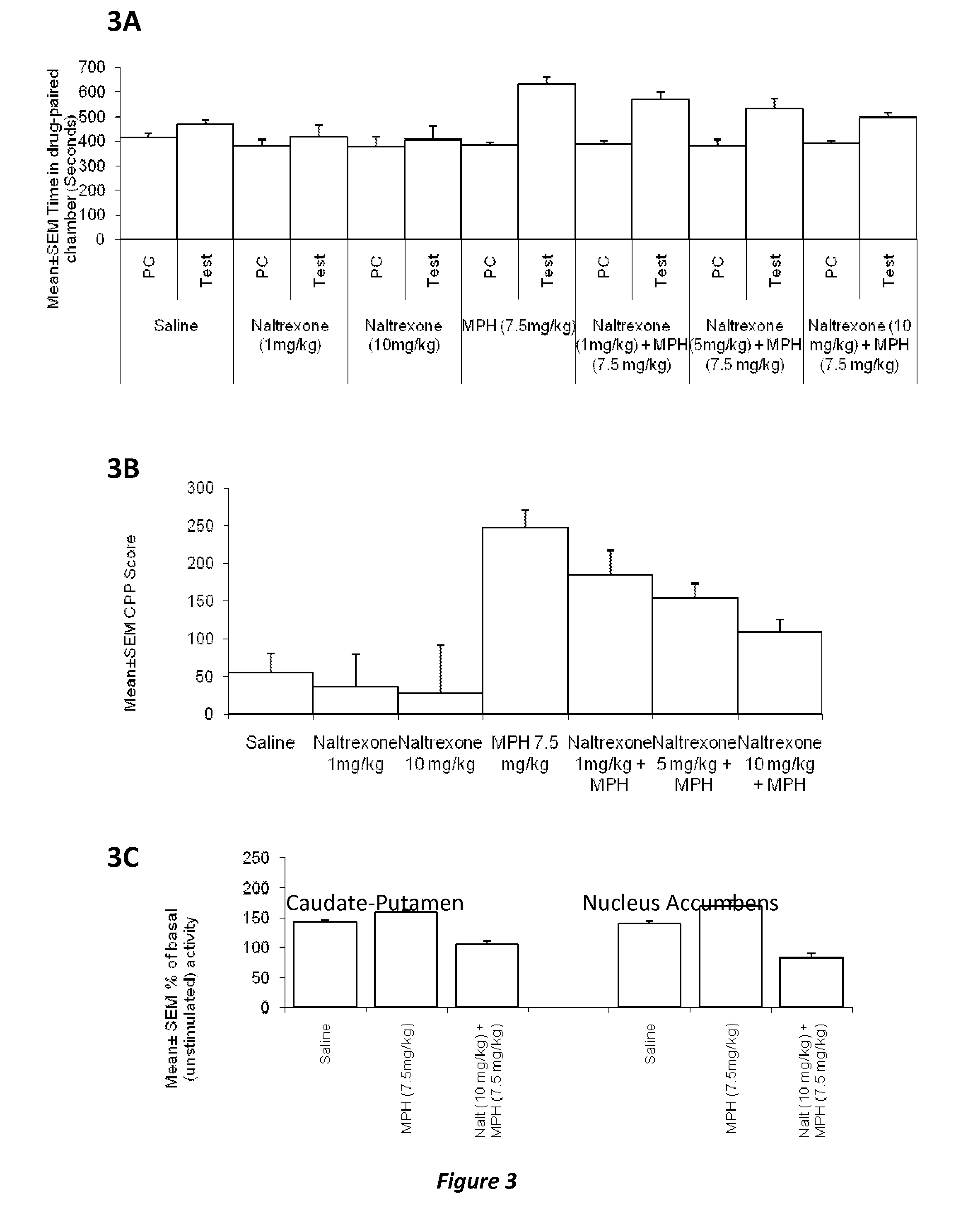
InactiveUS20120302590A1Decrease and lessening of aversionDecrease and lessening of and addictionBiocideNervous disorderNervous systemStimulant
Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject comprising, administering a therapeutic amount of the neurological stimulant and administering an antagonist of the kappa opioid receptor, to thereby reduce or prevent the development of aversion to the CNS stimulant in the subject. Also disclosed is a method of reducing or preventing the development of addiction to a CNS stimulant in a subject, comprising, administering the CNS stimulant and administering a mu opioid receptor antagonist to thereby reduce or prevent the development of addiction to the CNS stimulant in the subject. Also disclosed are pharmaceutical compositions comprising a central nervous system stimulant and an opioid receptor antagonist. Examples of central nervous system stimulants (such as methylphenidate) and opioid receptor antagonists (such as naltrexone) are provided.
Owner:THE GENERAL HOSPITAL CORP
Novel carbamate amino acid and peptide prodrugs of opiates and uses thereof
Carbamate linked prodrugs of meptazinol and other opioid analgesics are provided. The prodrug moiety may comprise a single amino acid or short peptide. Additionally, the present invention relates to methods for reducing gastrointestinal side effects in a subject, the gastrointestinal side effects being associated with the administration of an opioid analgesic. The methods comprise orally administering an opioid prodrug or pharmaceutically acceptable salt thereof to a subject, wherein the opioid pro-drug is comprised of an opioid analgesic covalently bonded through a carbamate linkage to a prodrug moiety, and wherein upon oral administration, the prodrug or pharmaceutically acceptable salt minimizes at least one gastrointestinal side effect associated with oral administration of the opioid analgesic alone. Compositions for use with the method are also provided.
Owner:SHIRE PLC
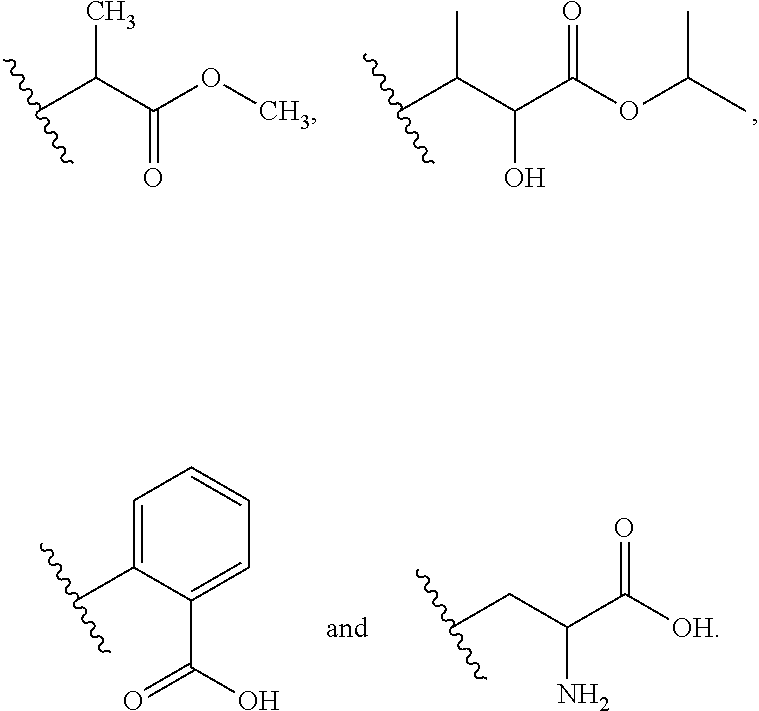
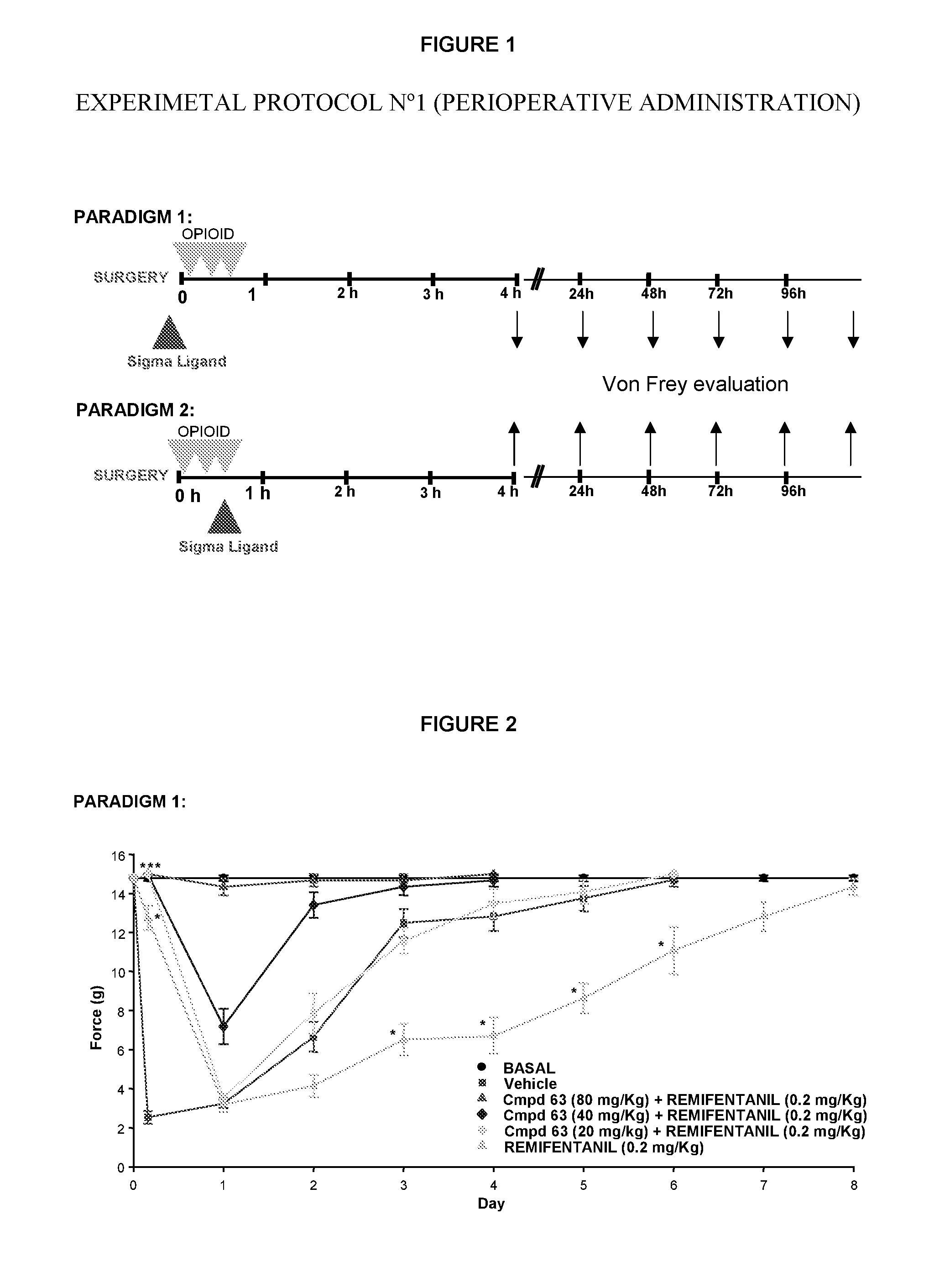
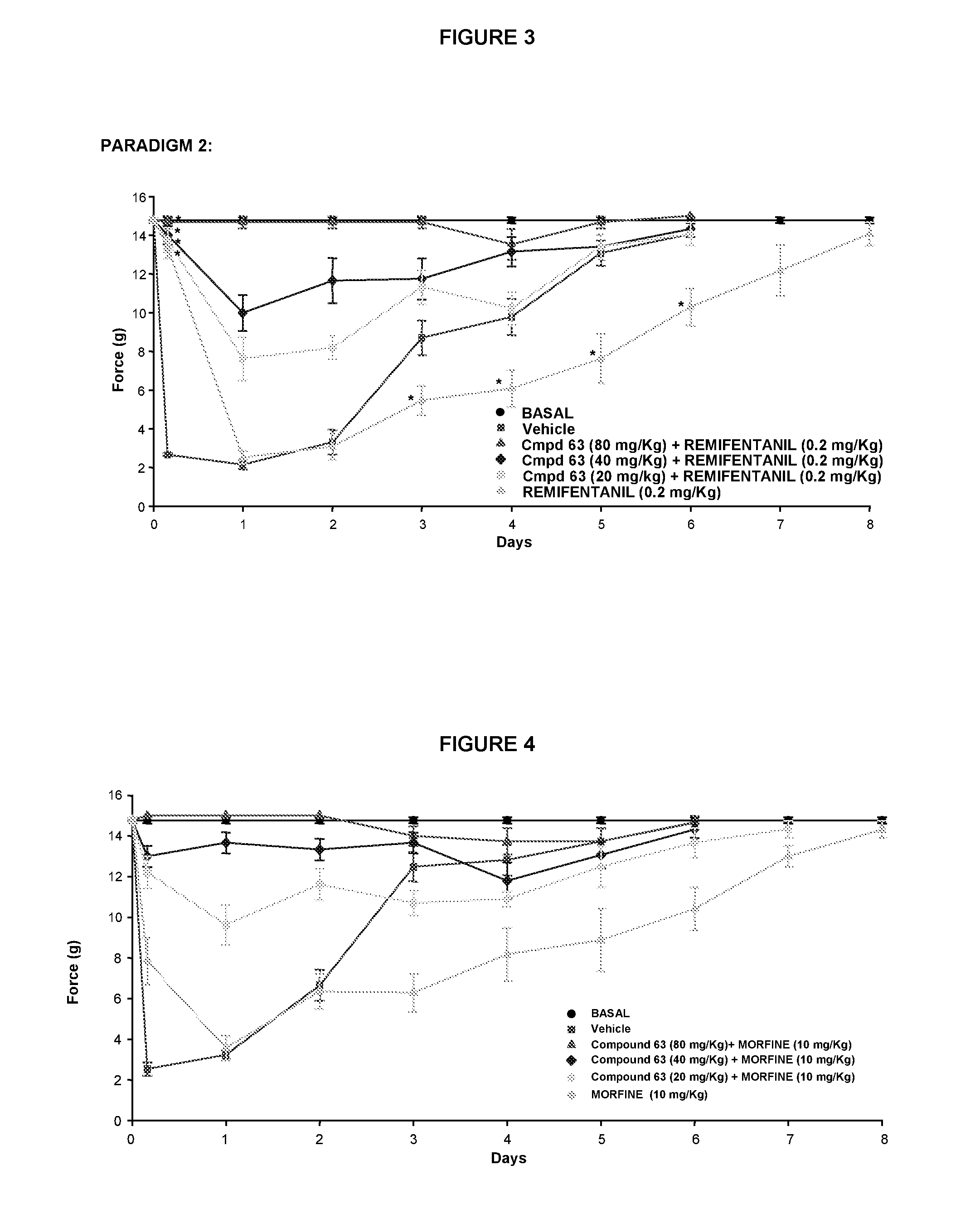
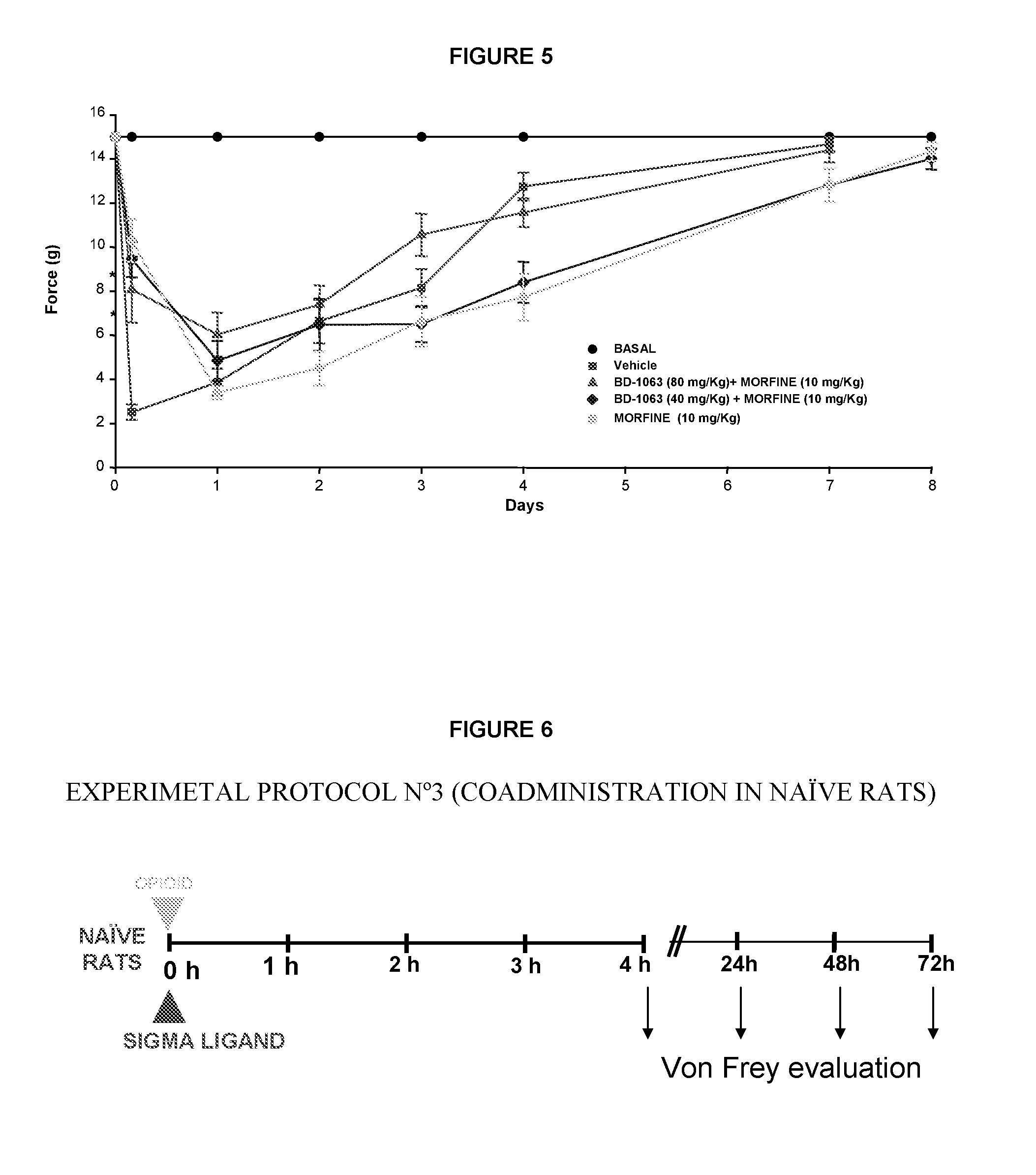
Use of sigma ligands in opioid-induced hyperalgesia
InactiveUS20130158033A1Organic active ingredientsNervous disorderMedicineOpioid-induced hyperalgesia
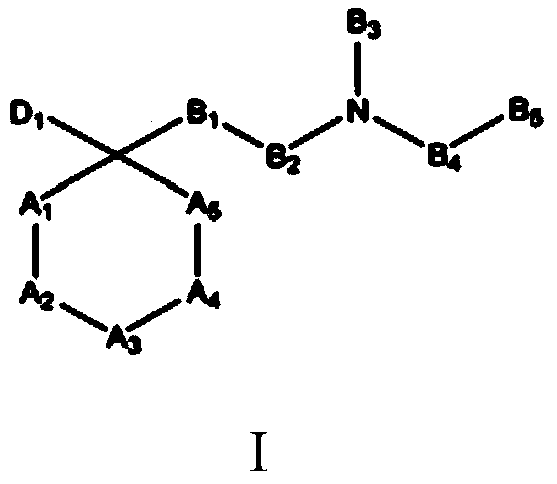
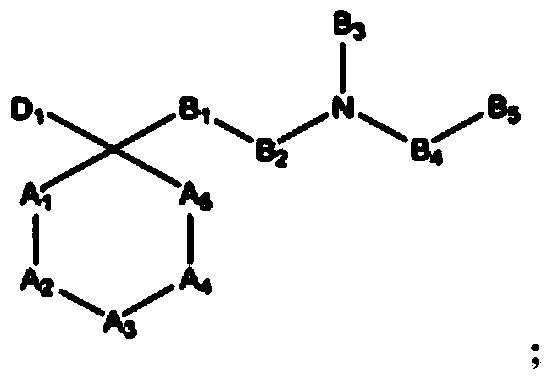
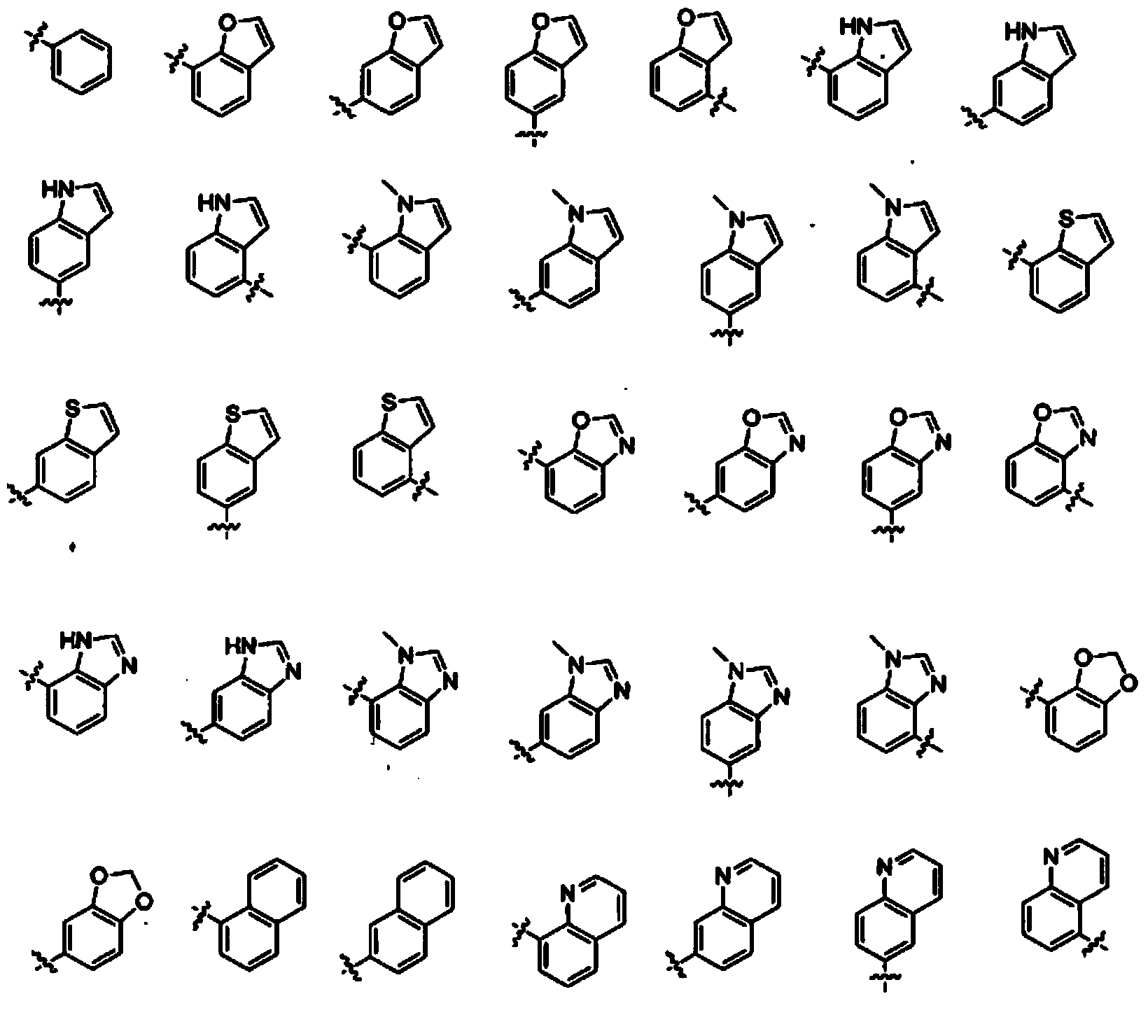
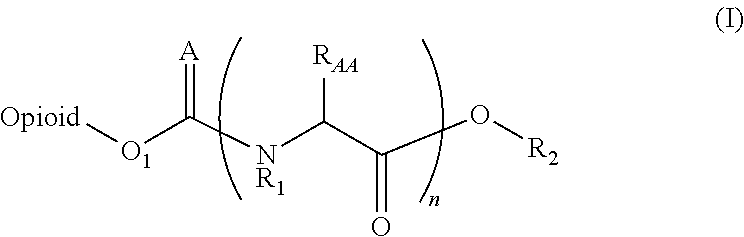
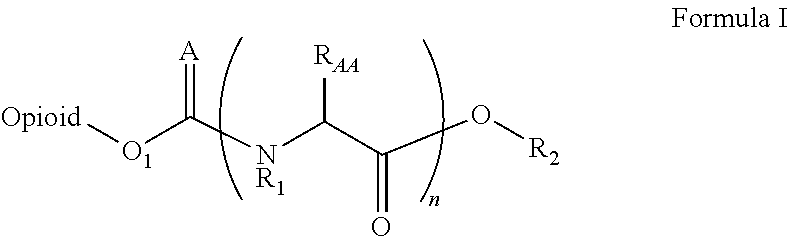
The invention refers to the use of a sigma ligand, particularly a sigma ligand of formula (I) to prevent and / or treat opioid-induced hyperalgesia (OIH) associated to opioid therapy.
Owner:LAB DEL DR ESTEVE SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com