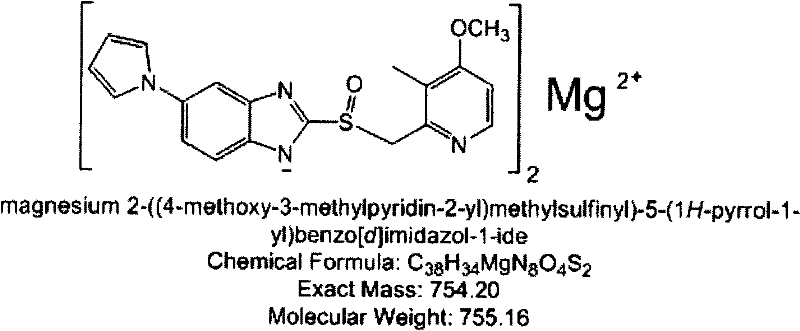
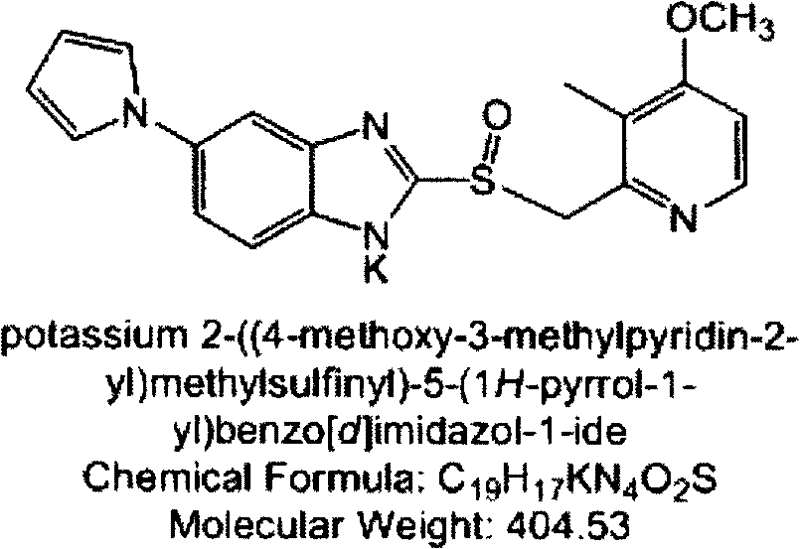
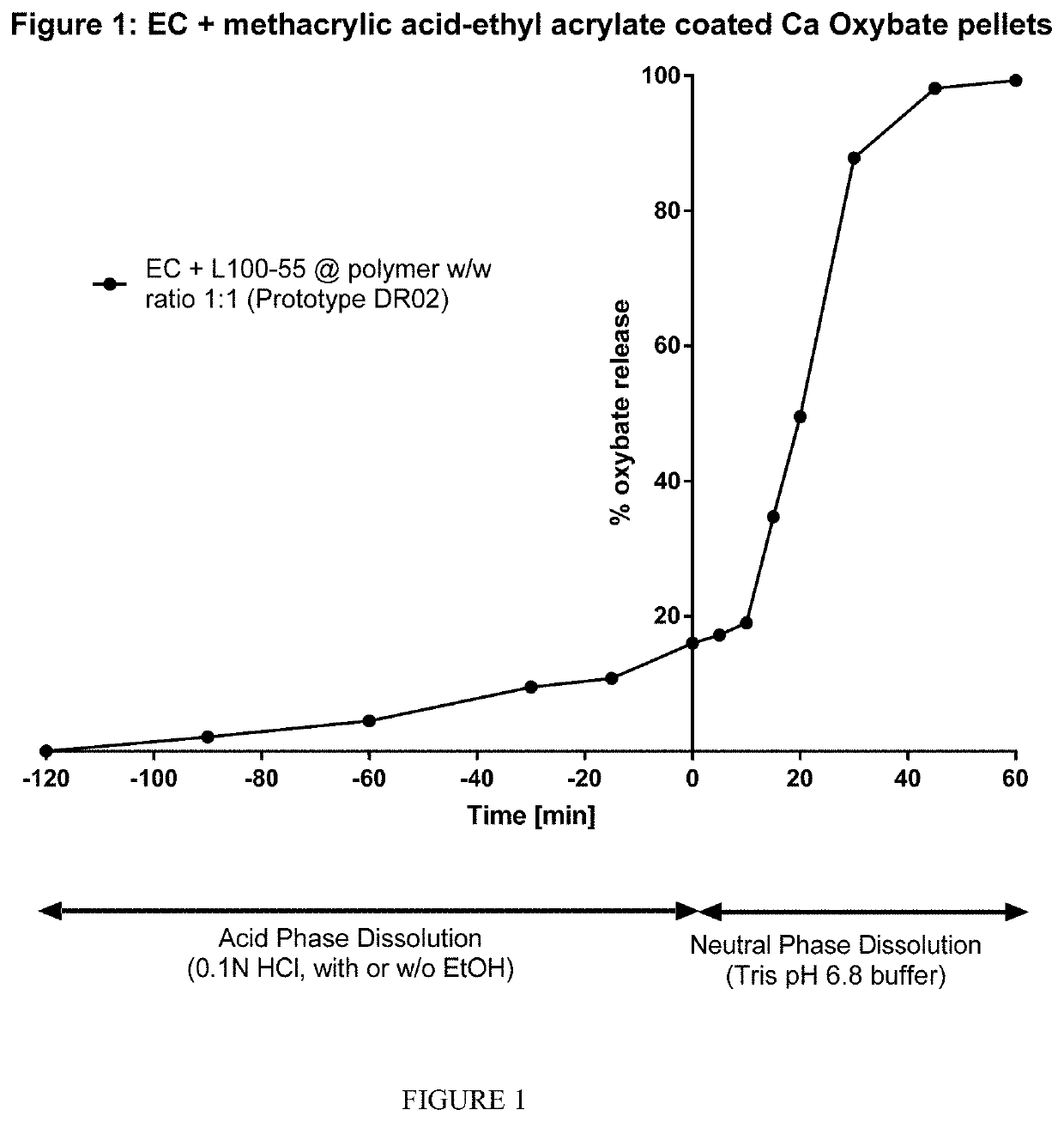
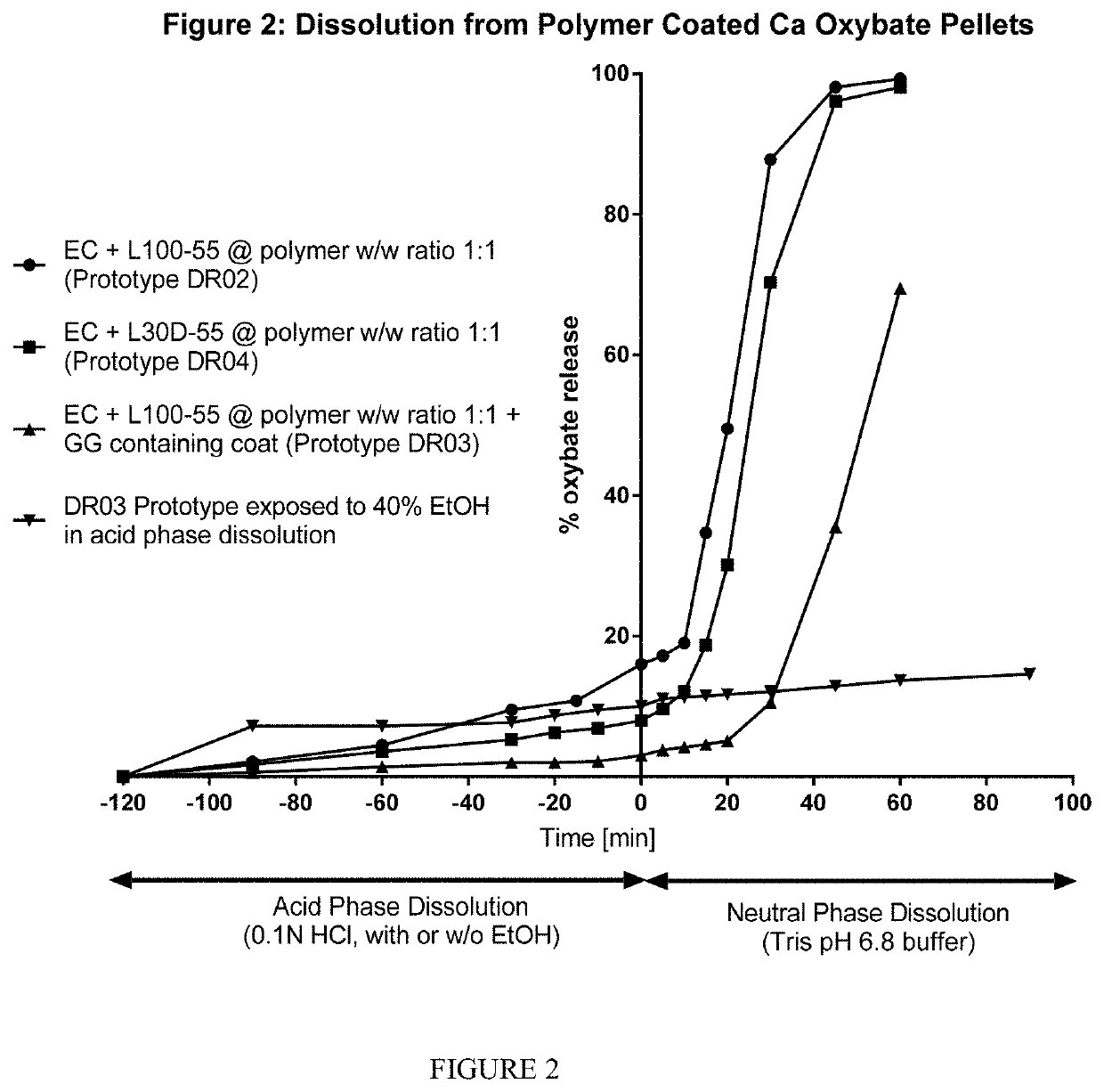
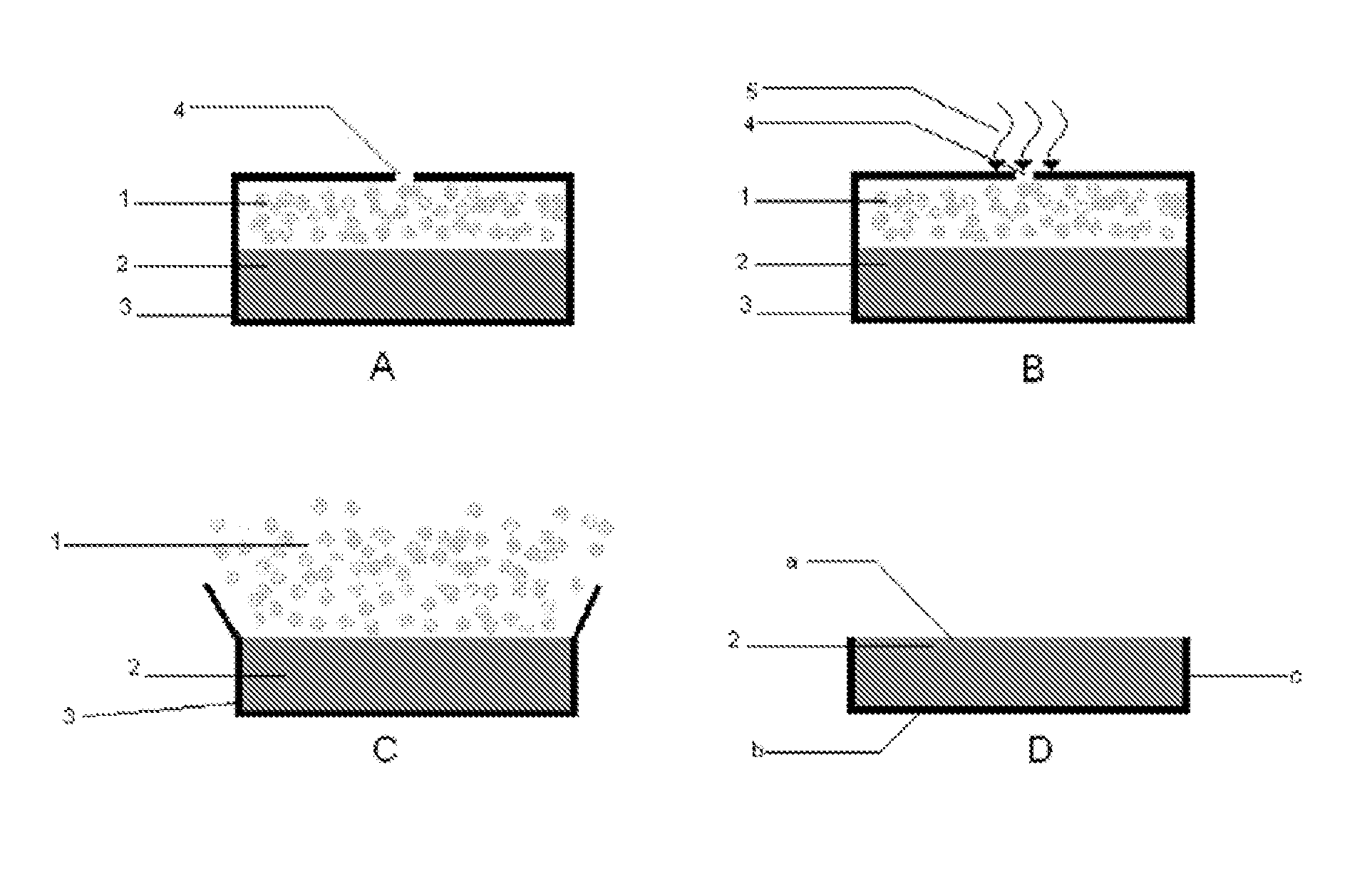
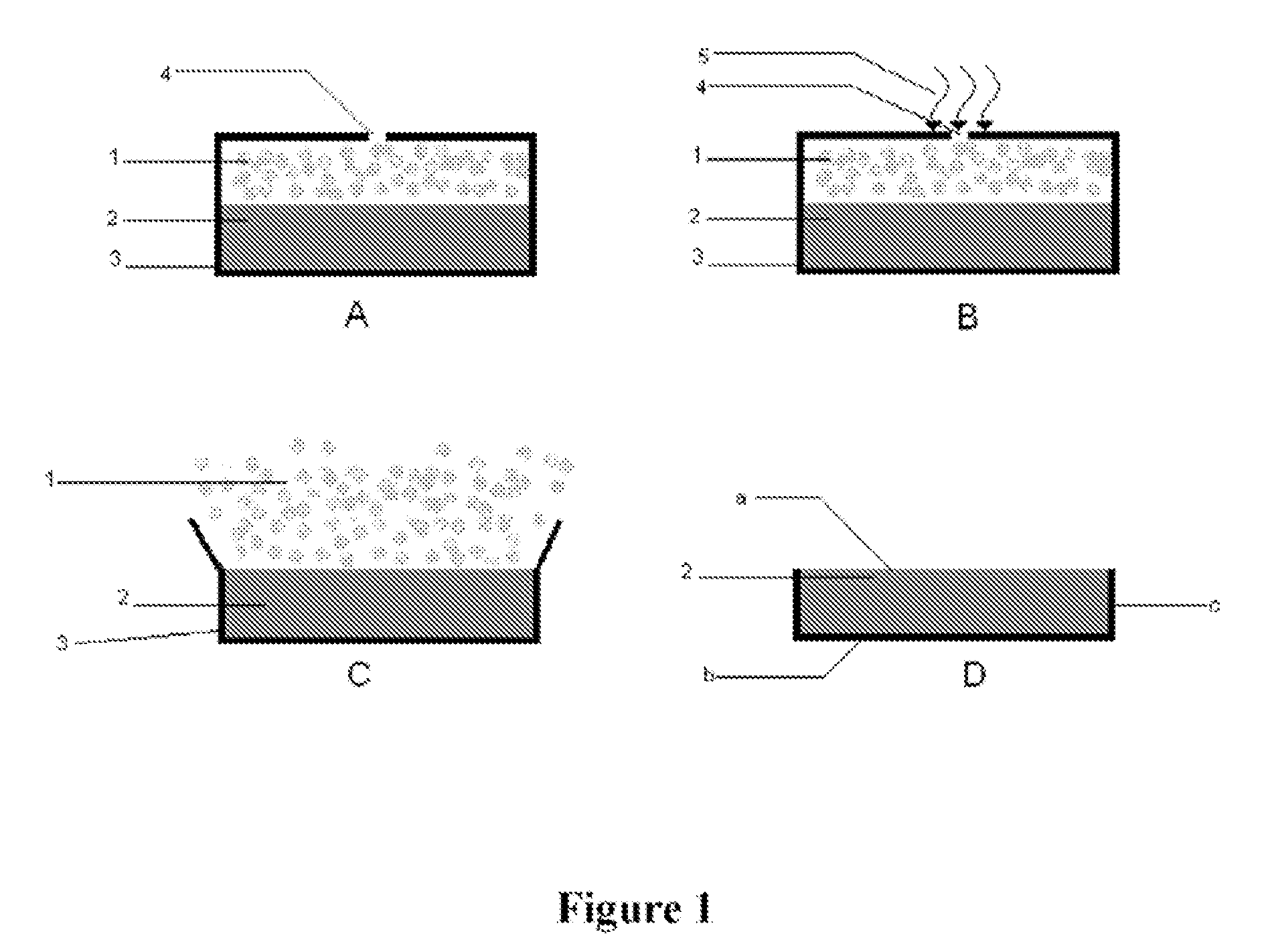
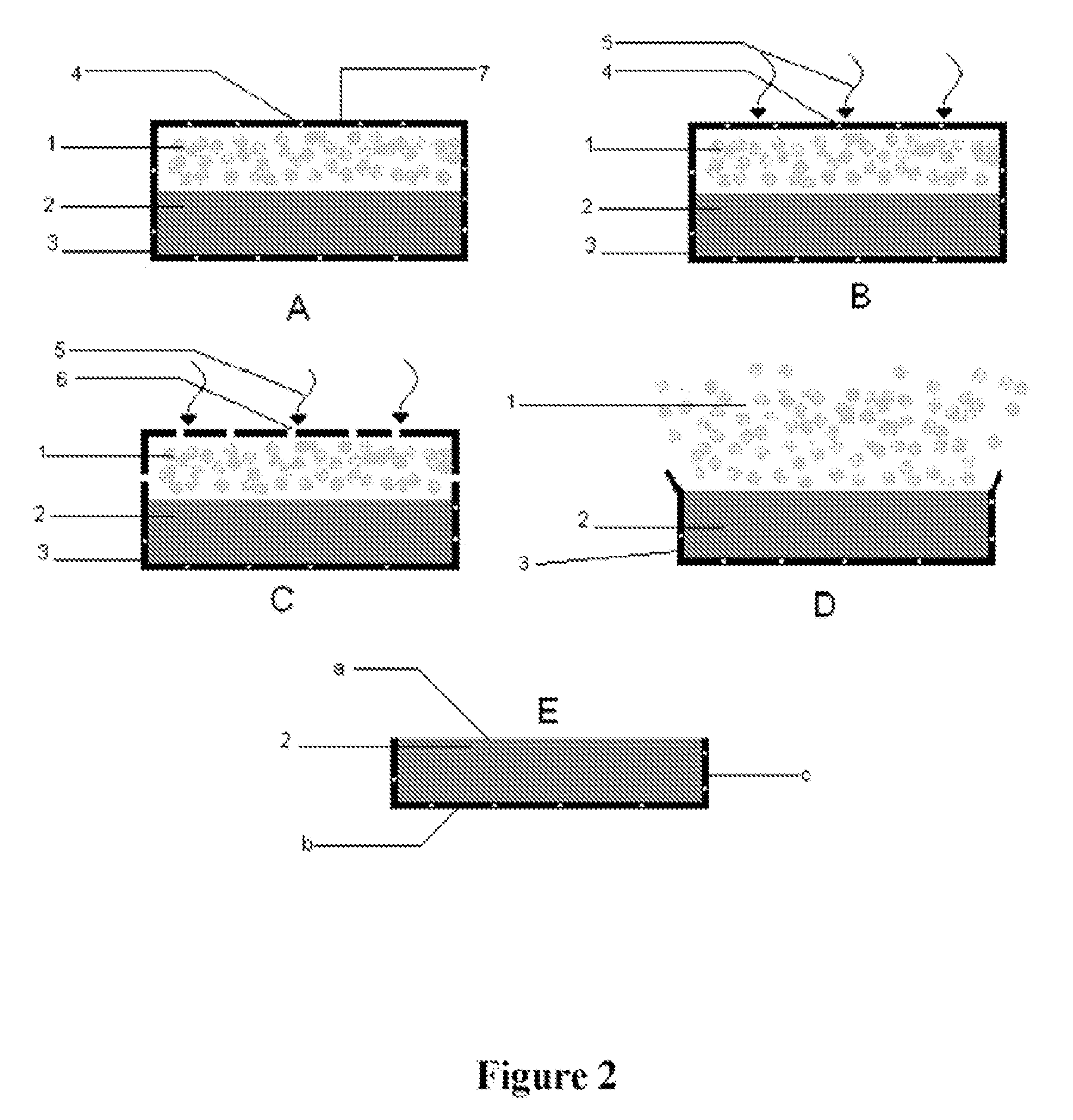
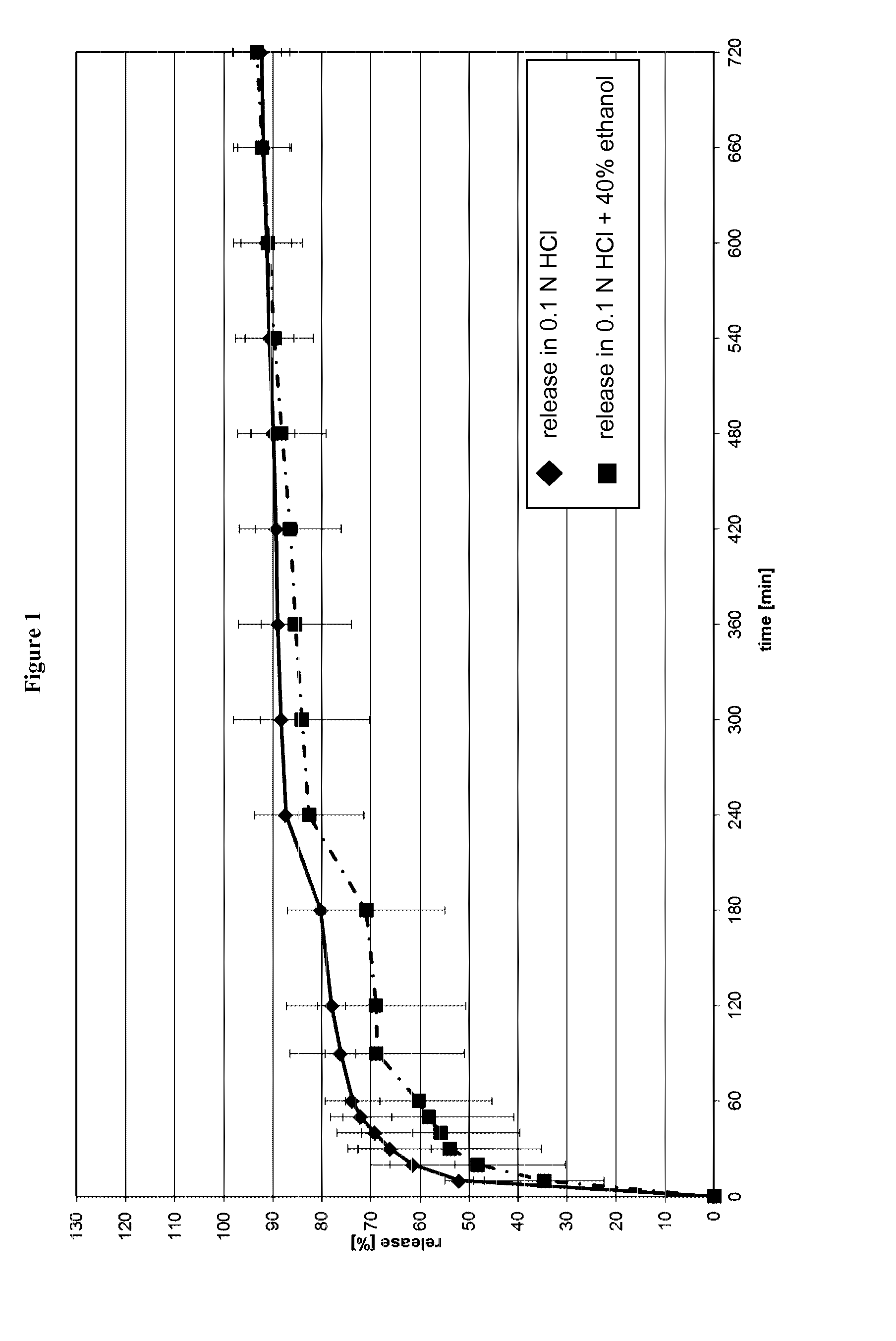
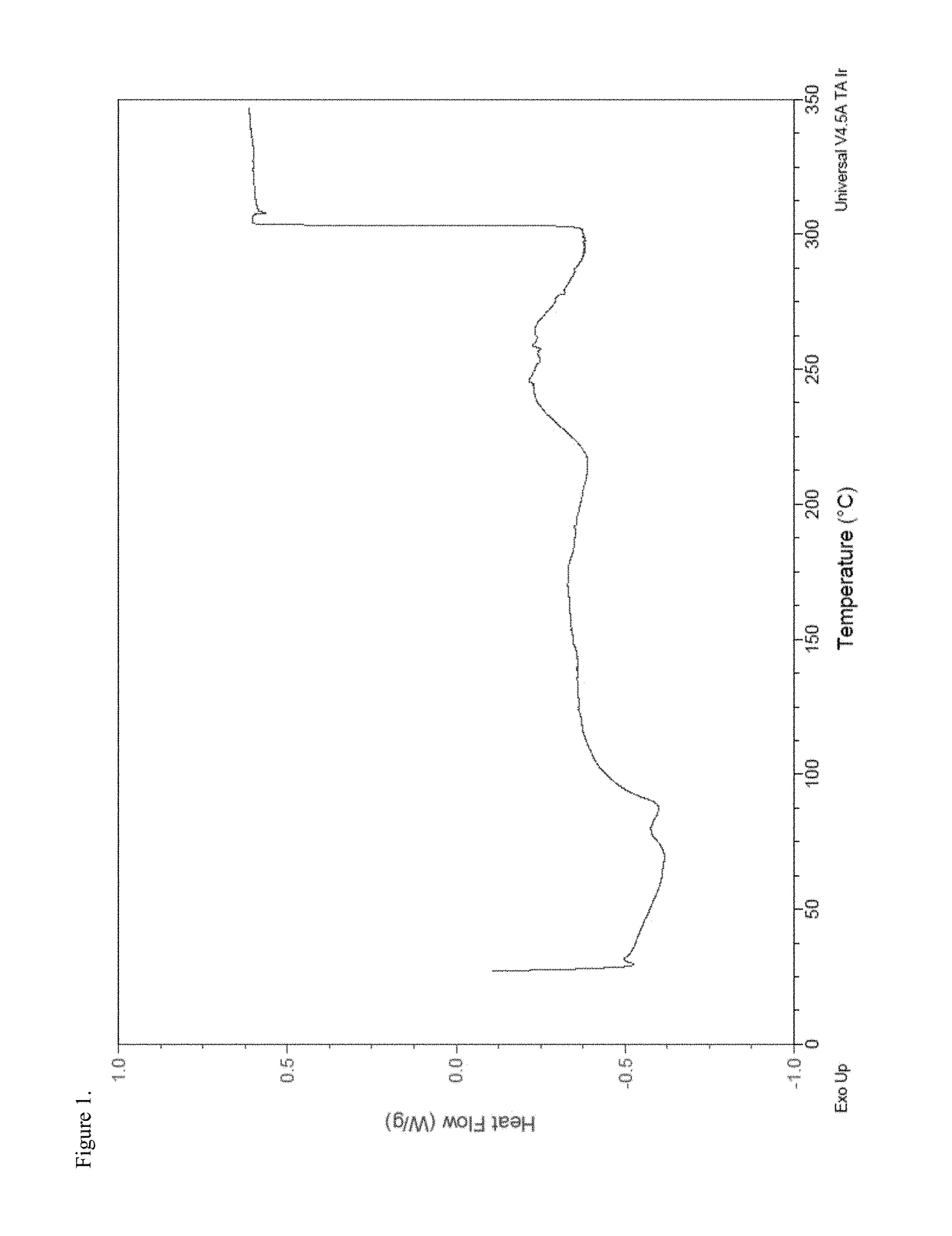
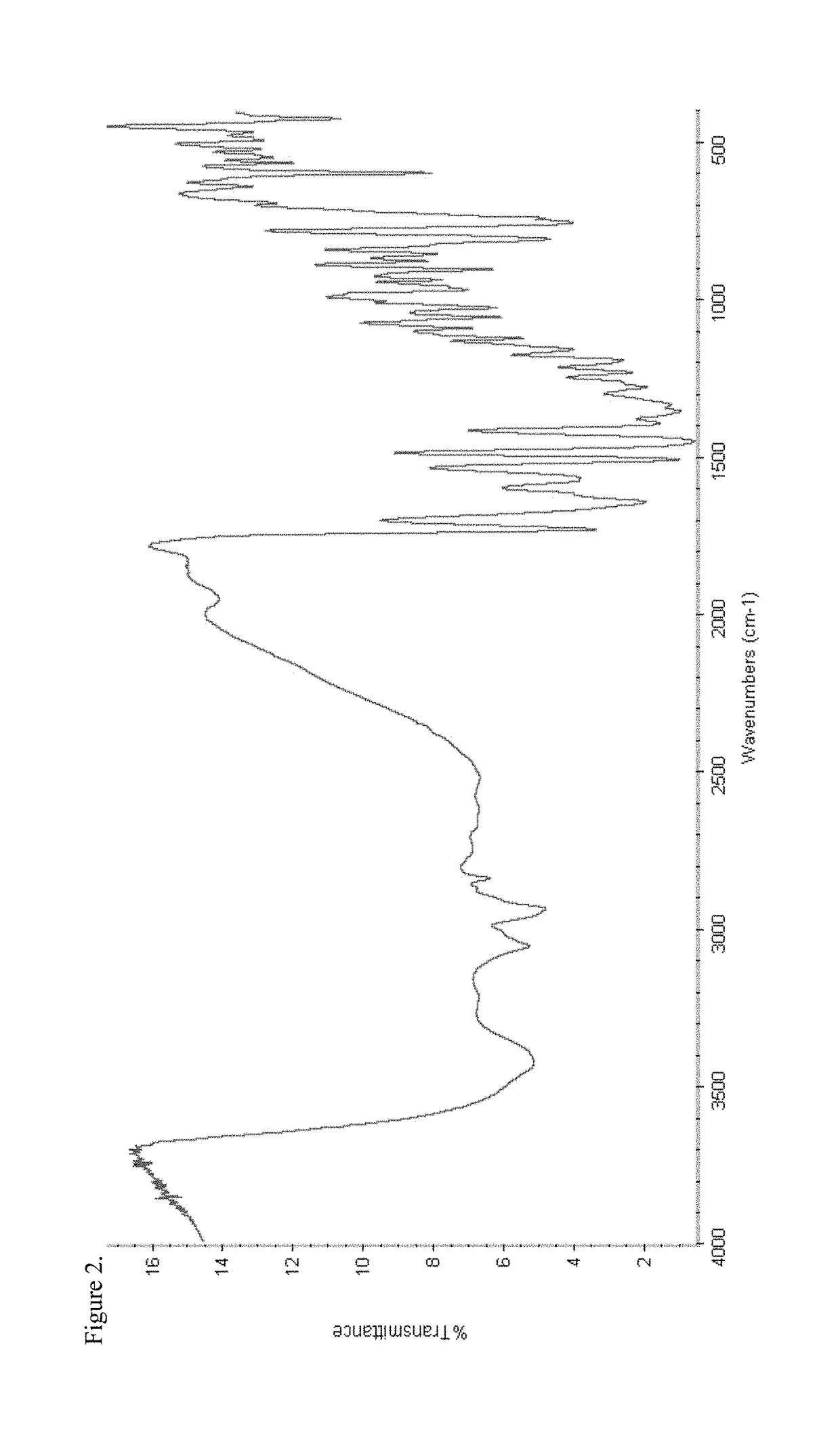
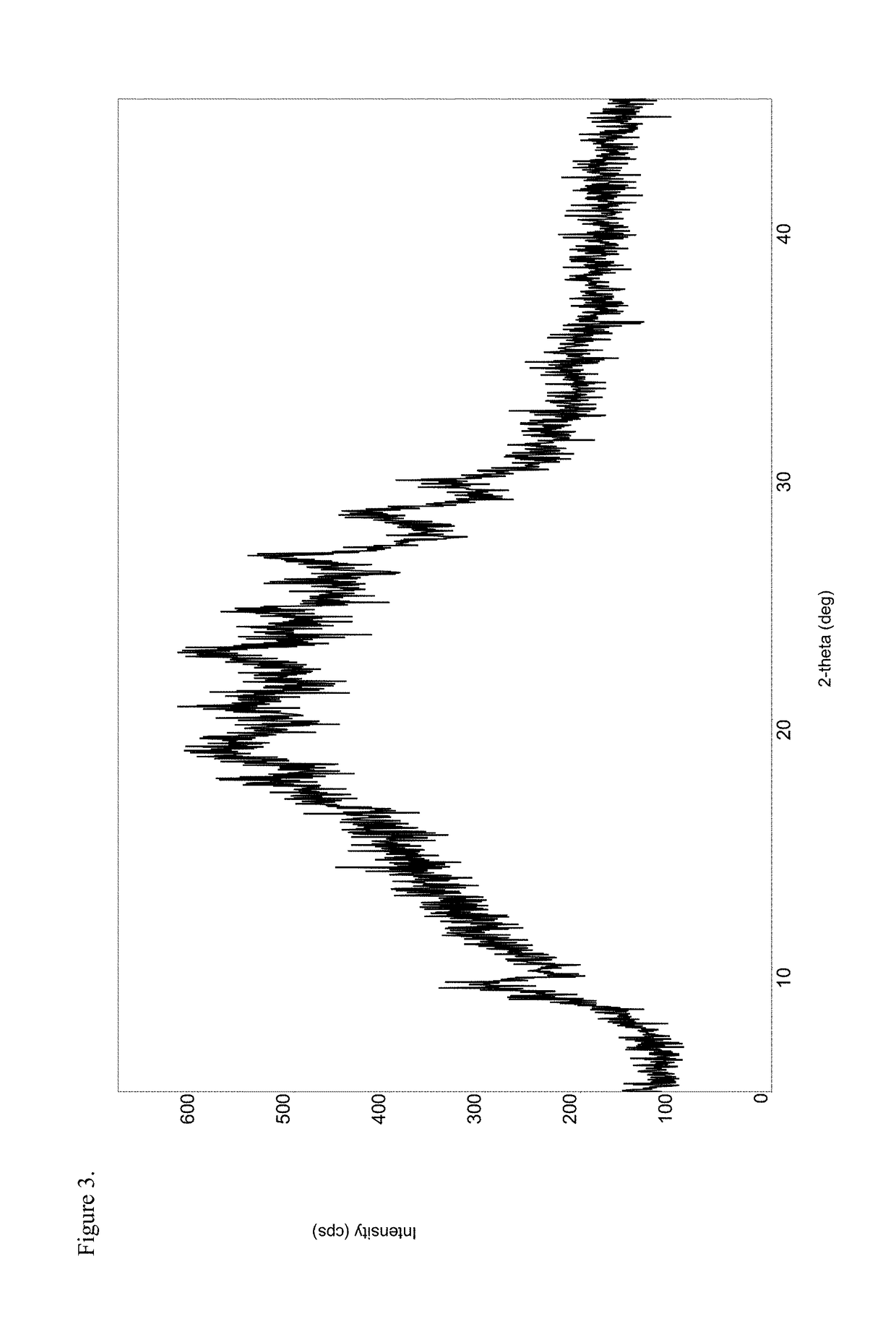
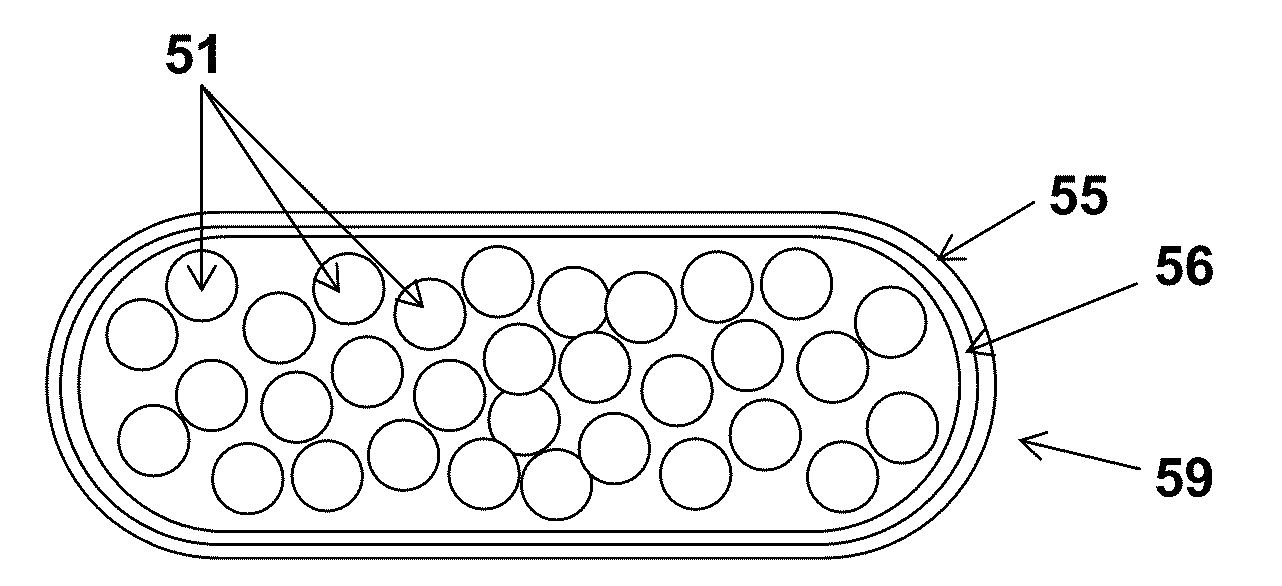
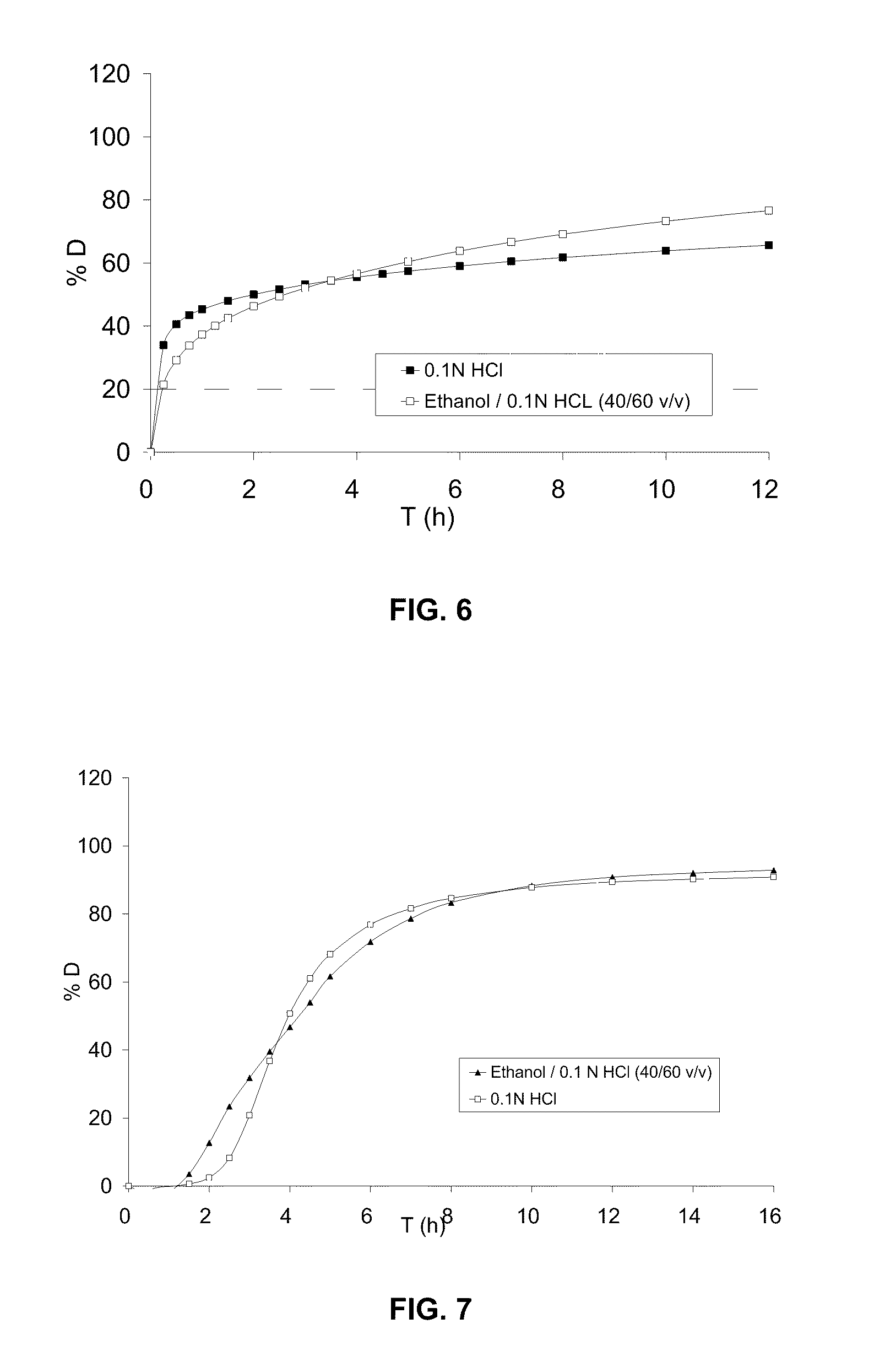
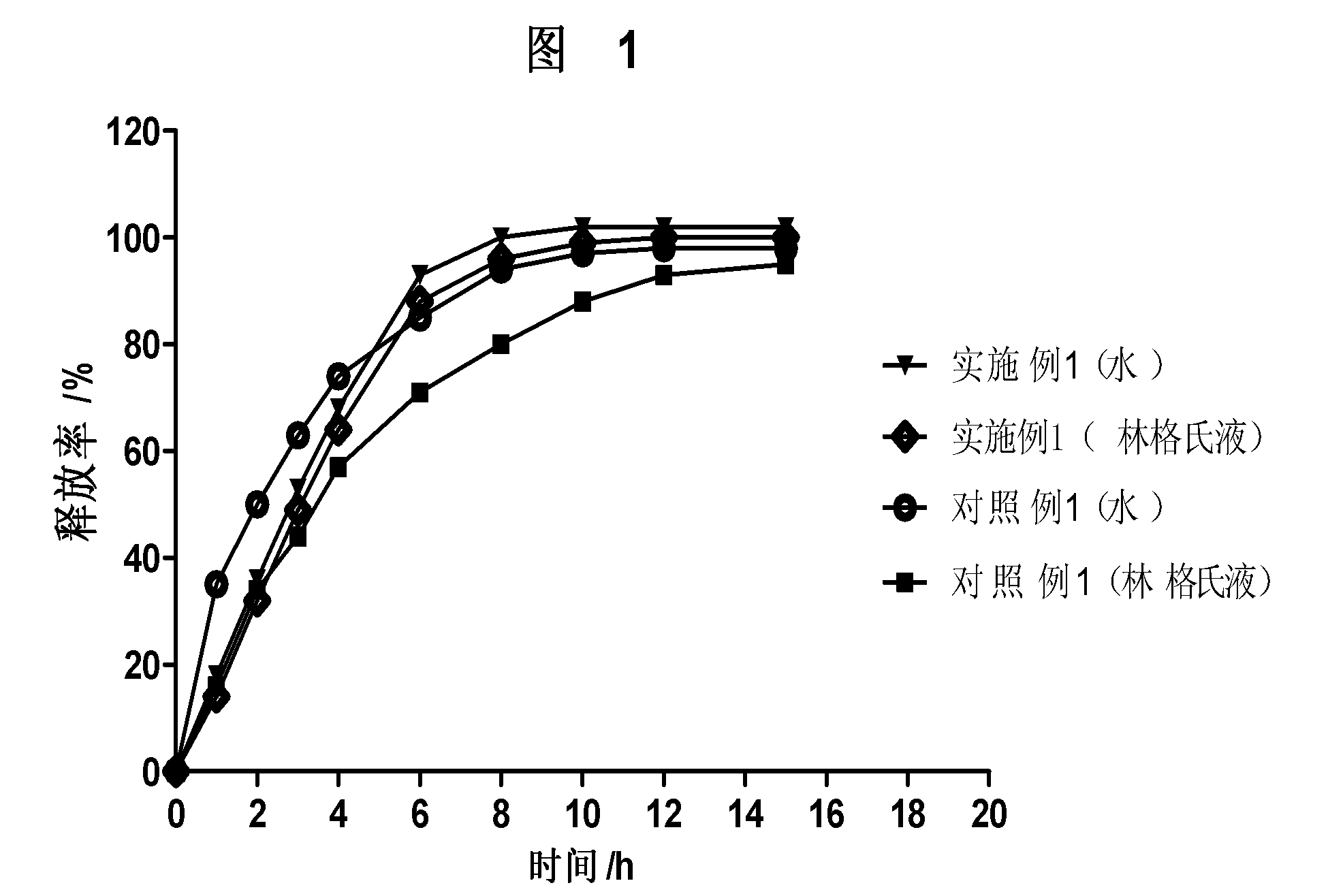
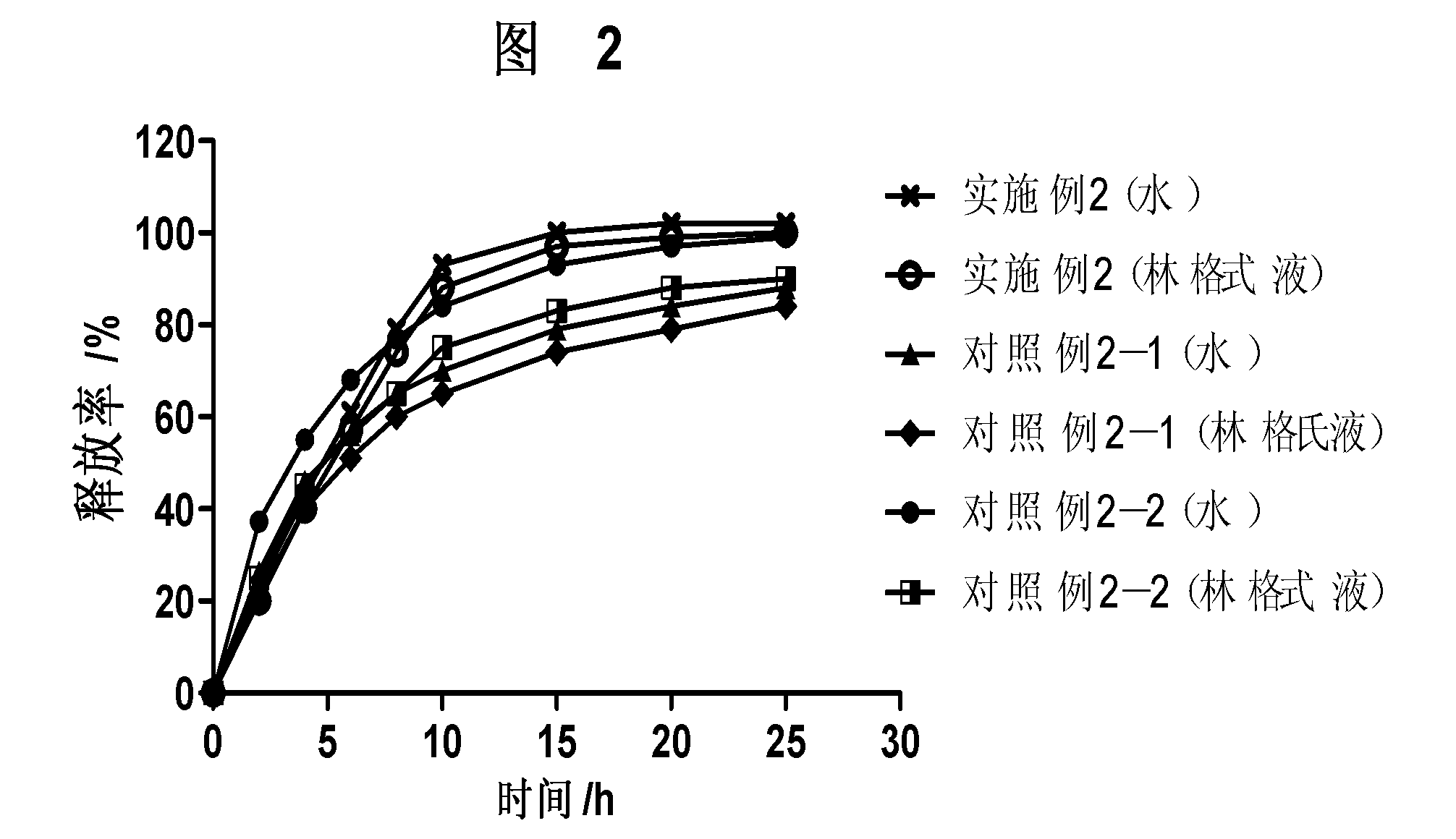
Patents
Literature
70 results about "Dose dumping" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
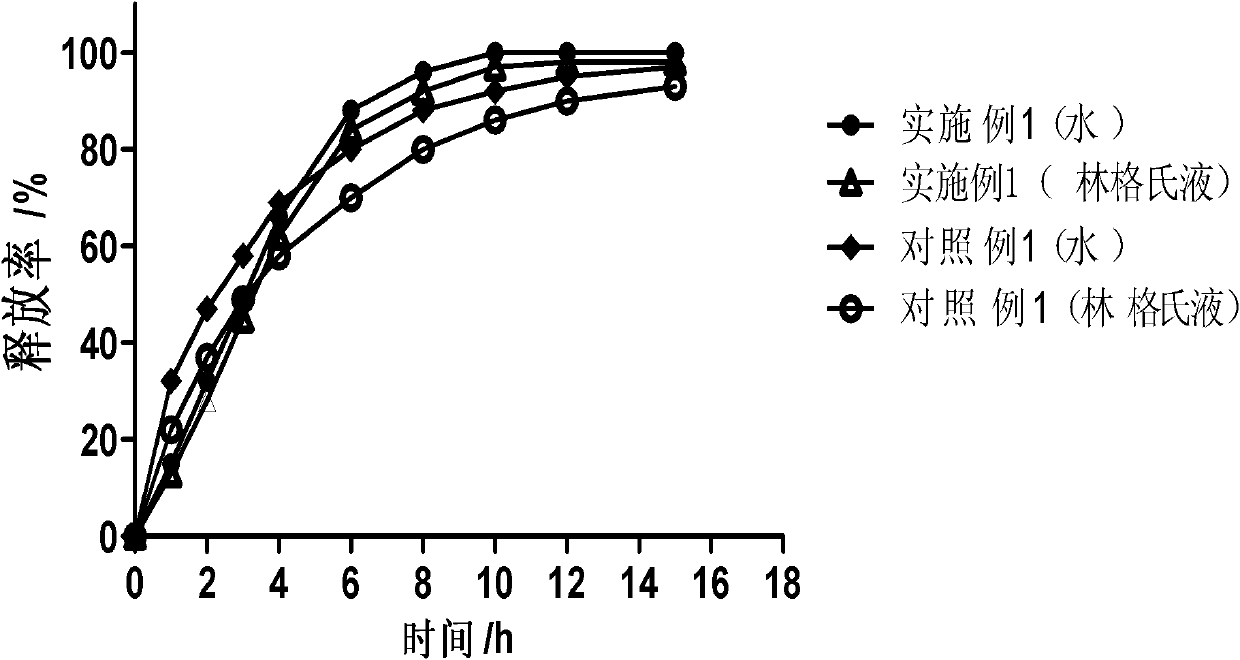
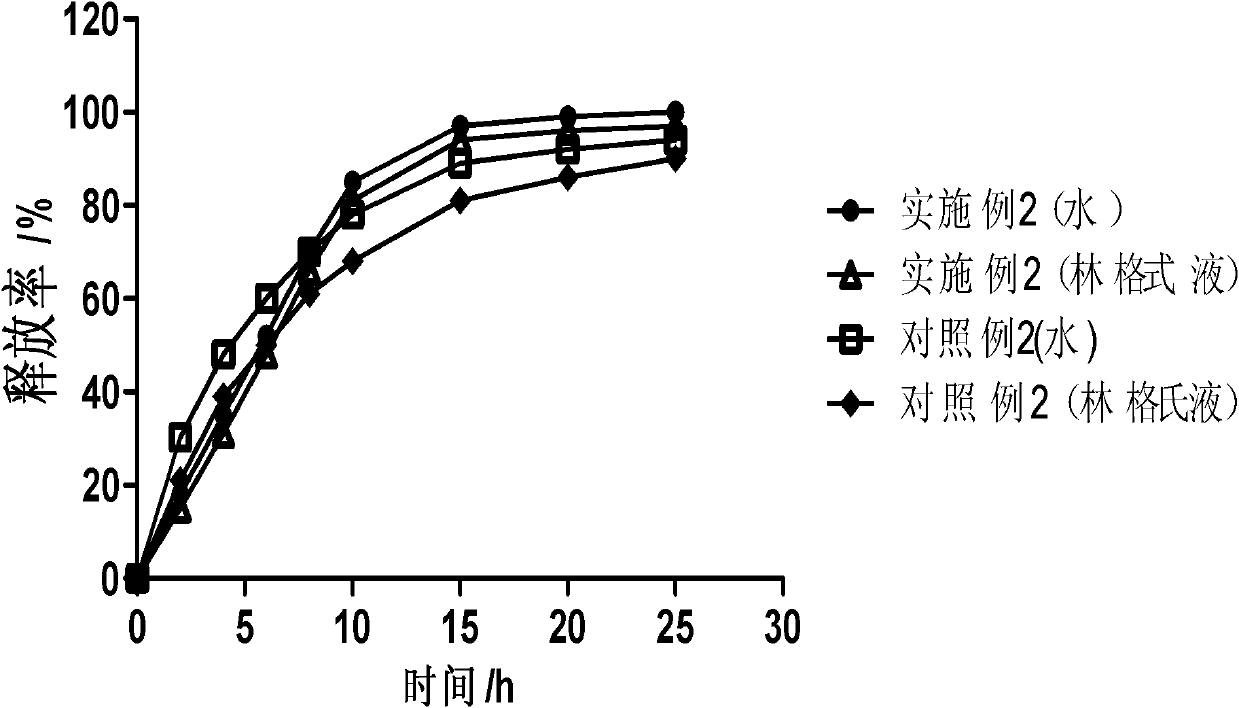
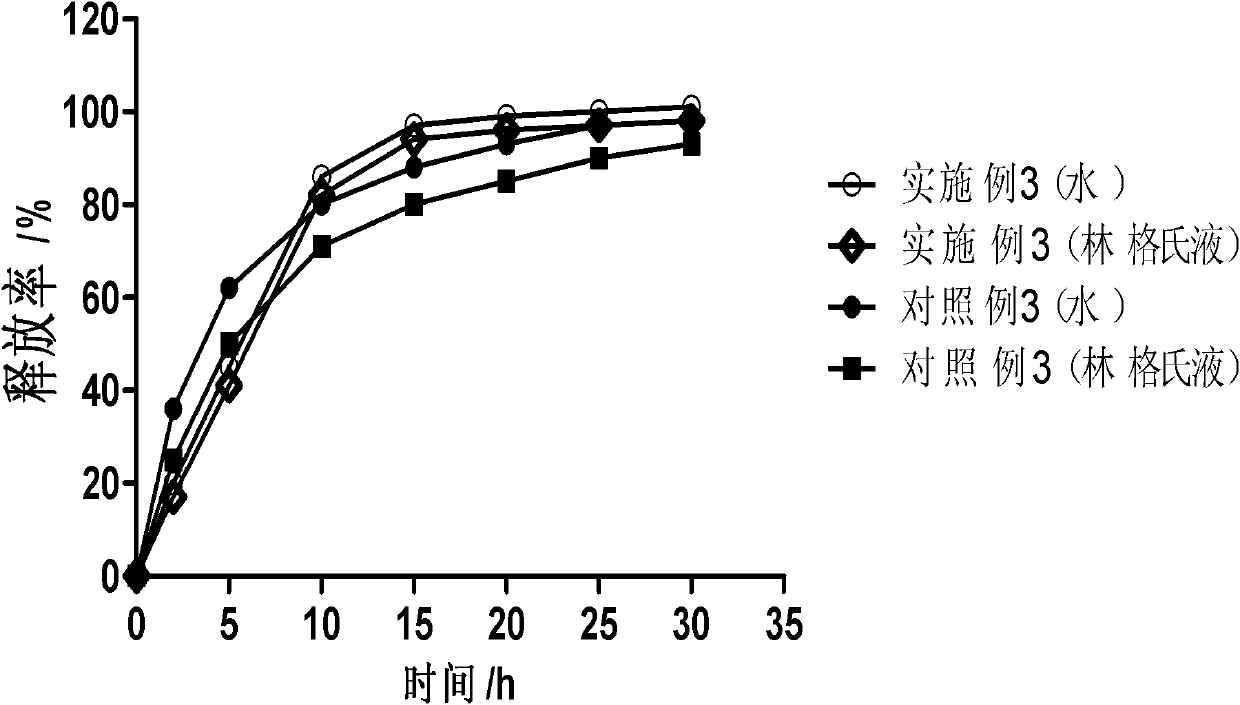
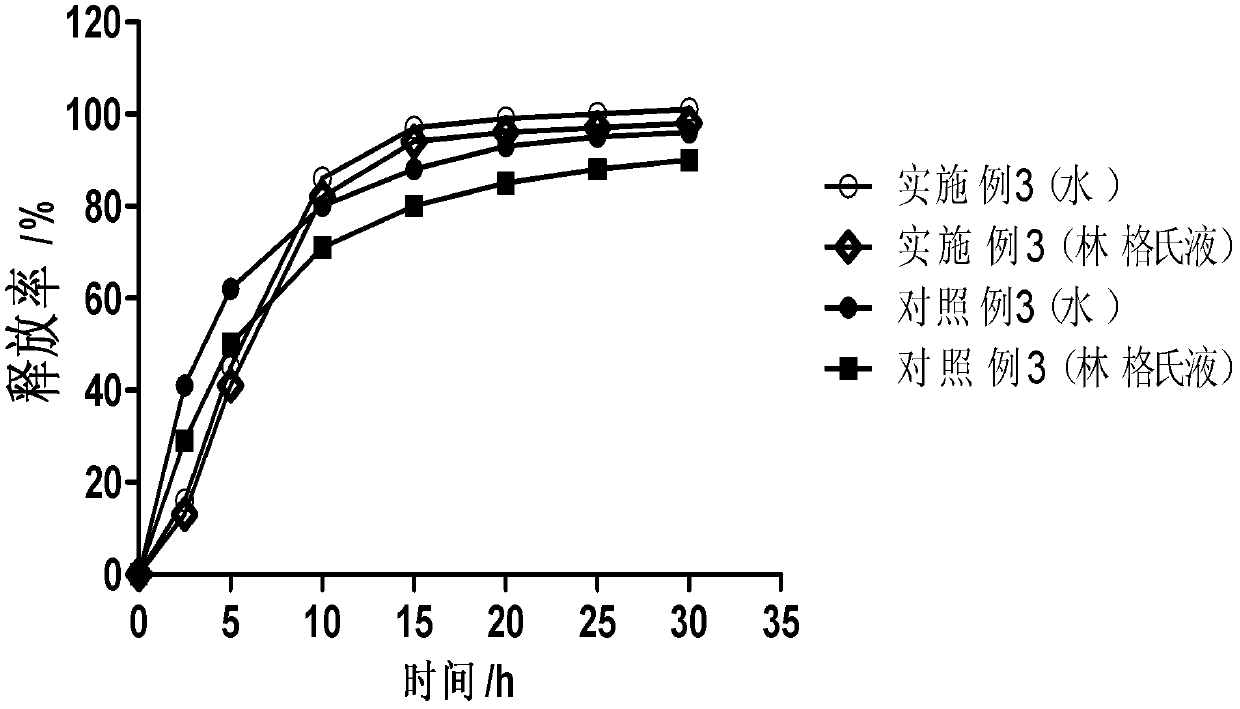
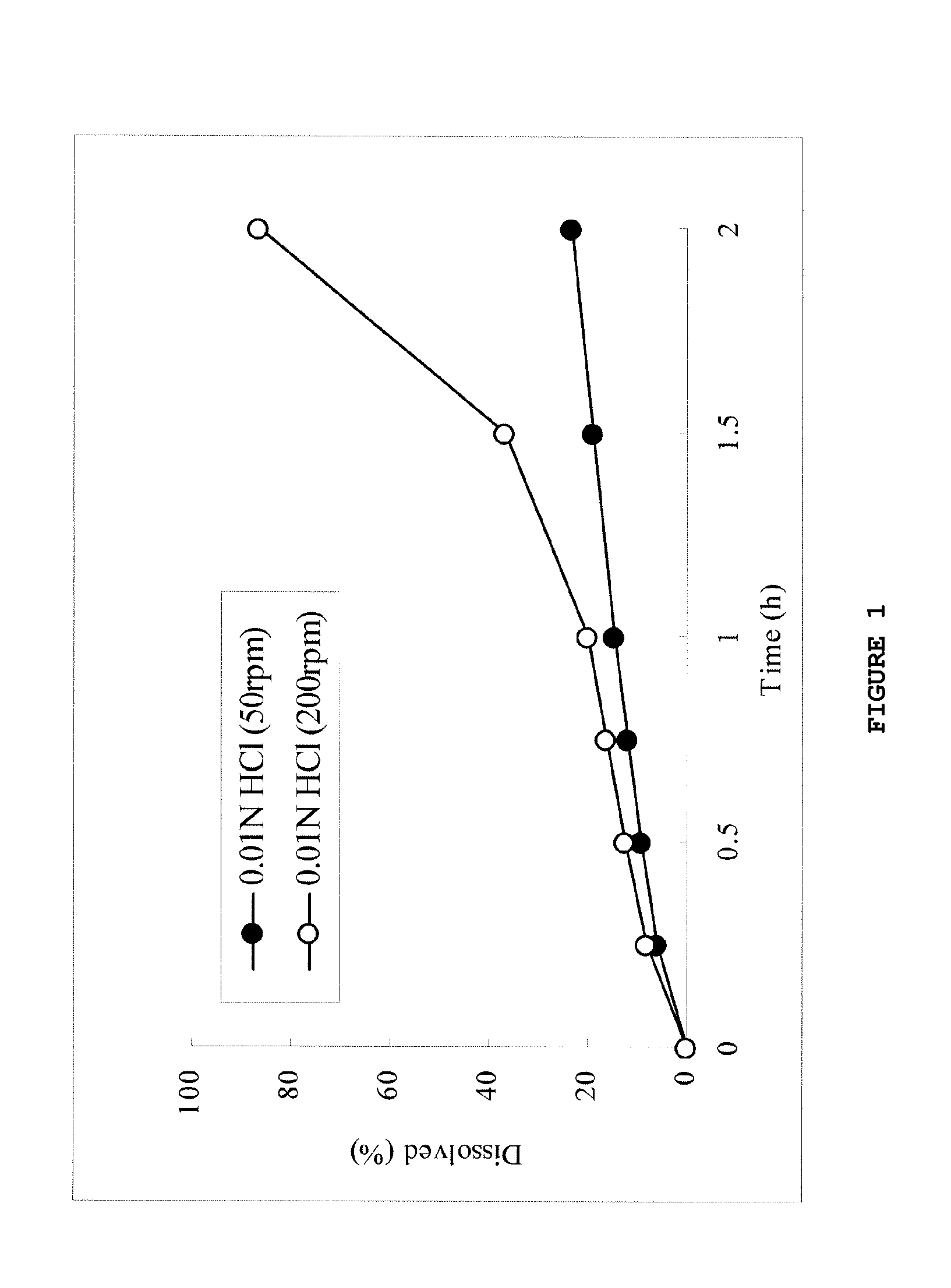
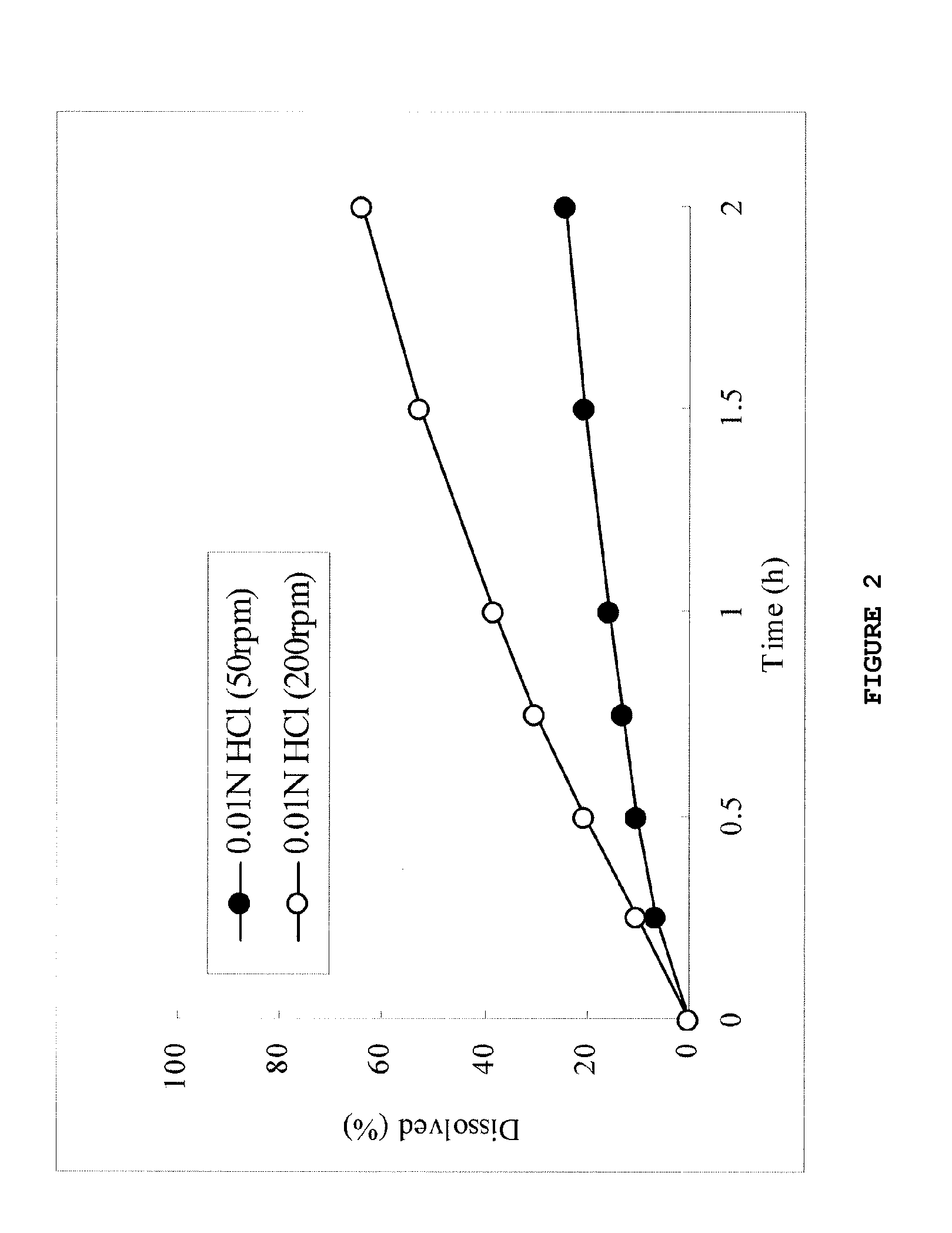
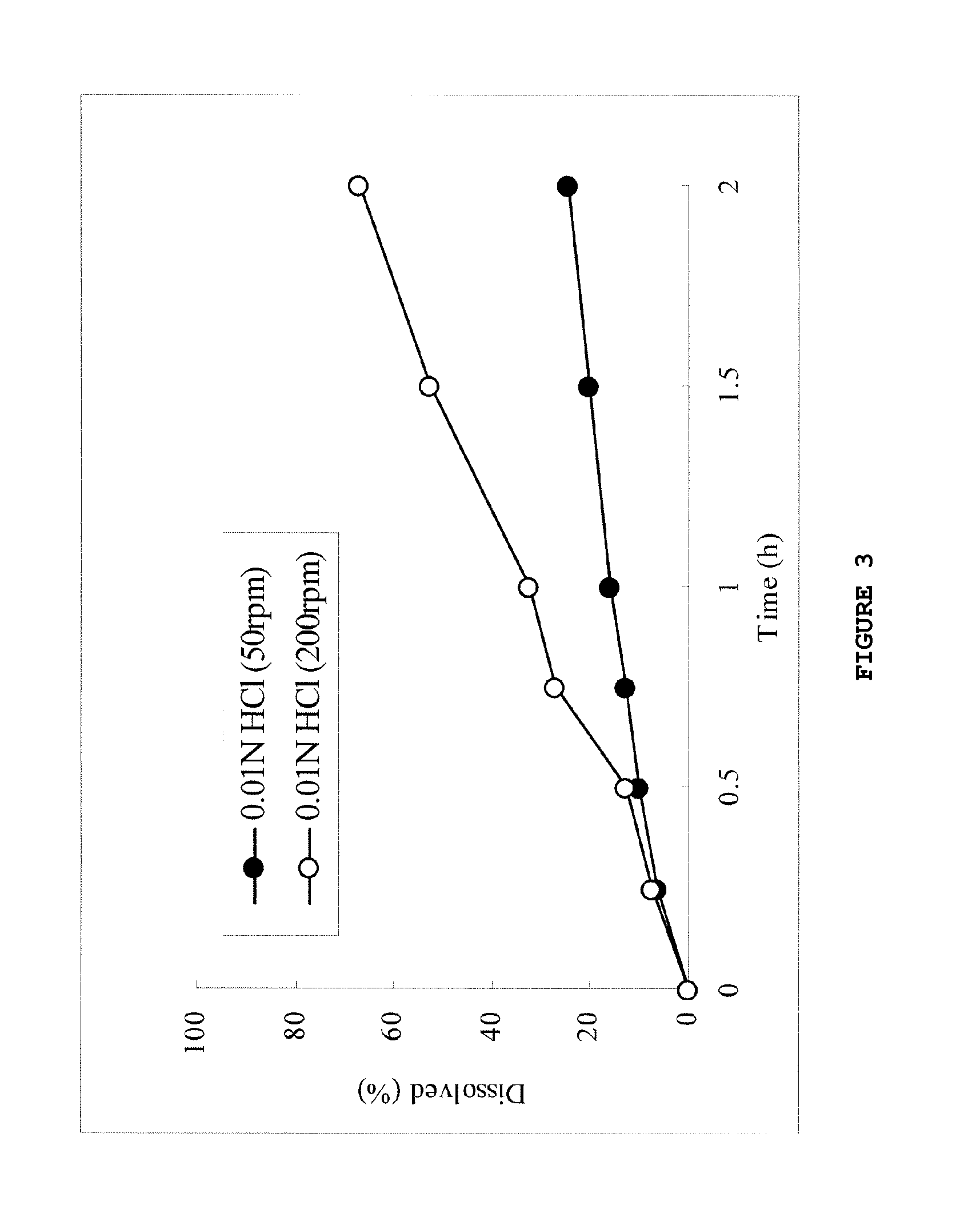
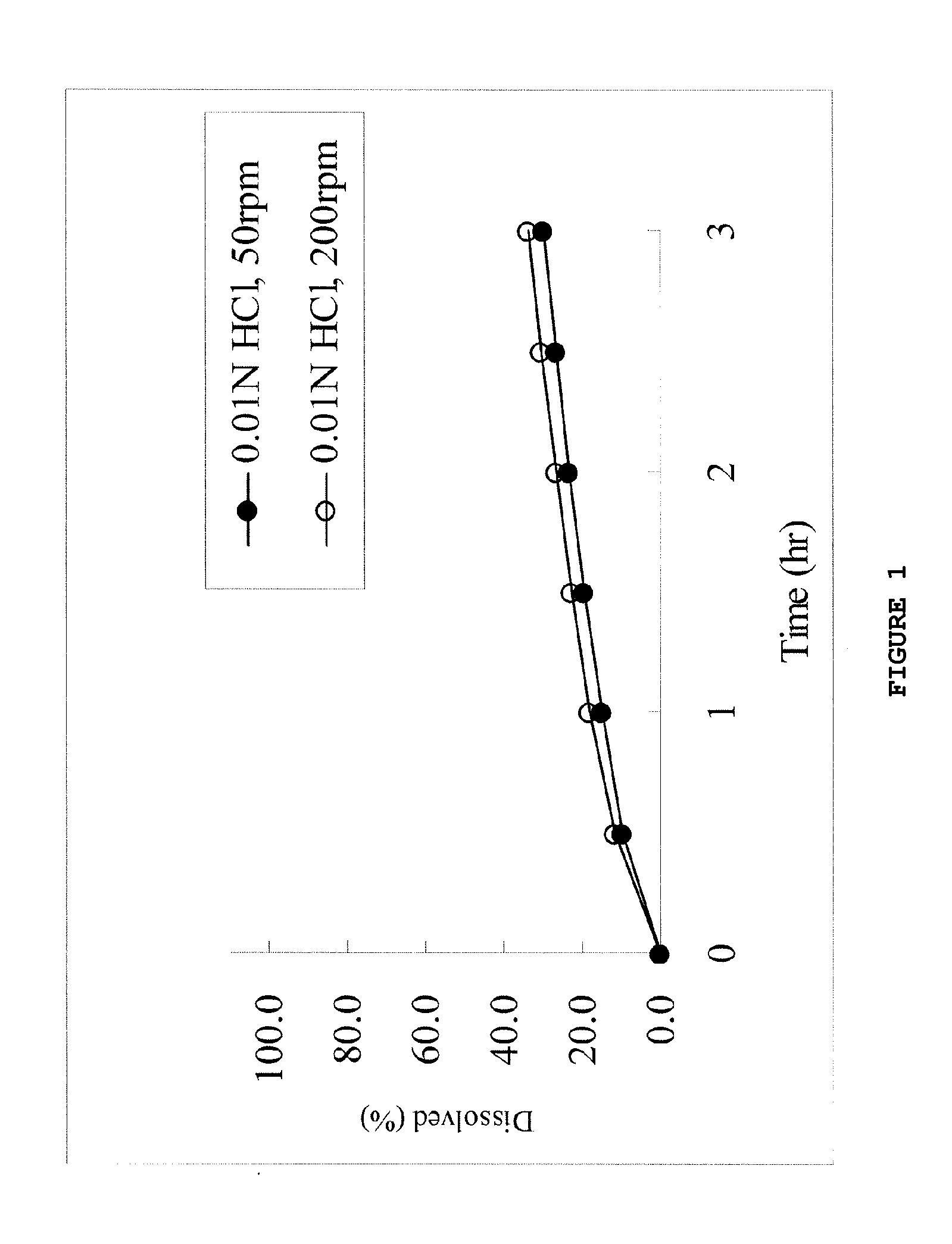
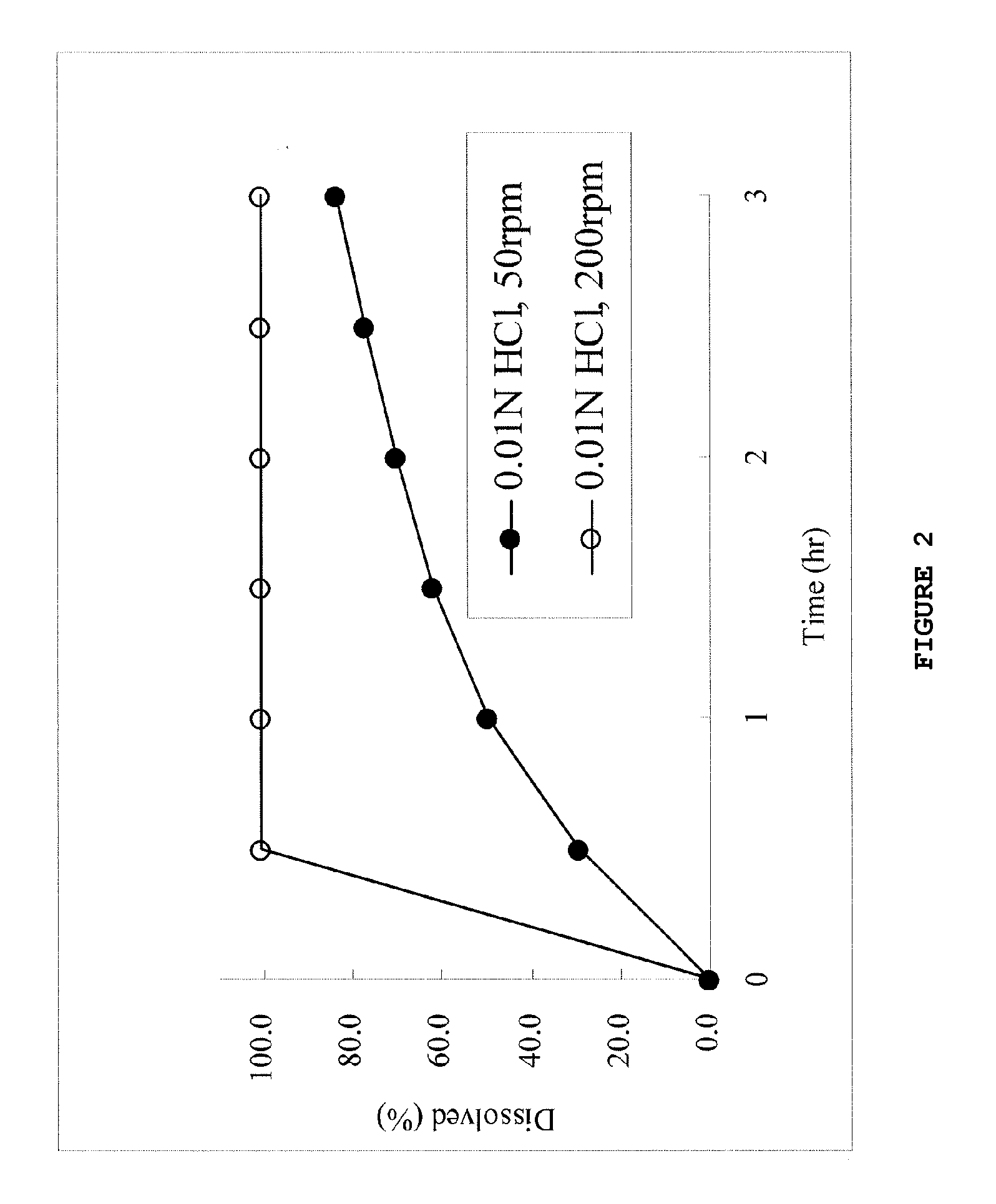
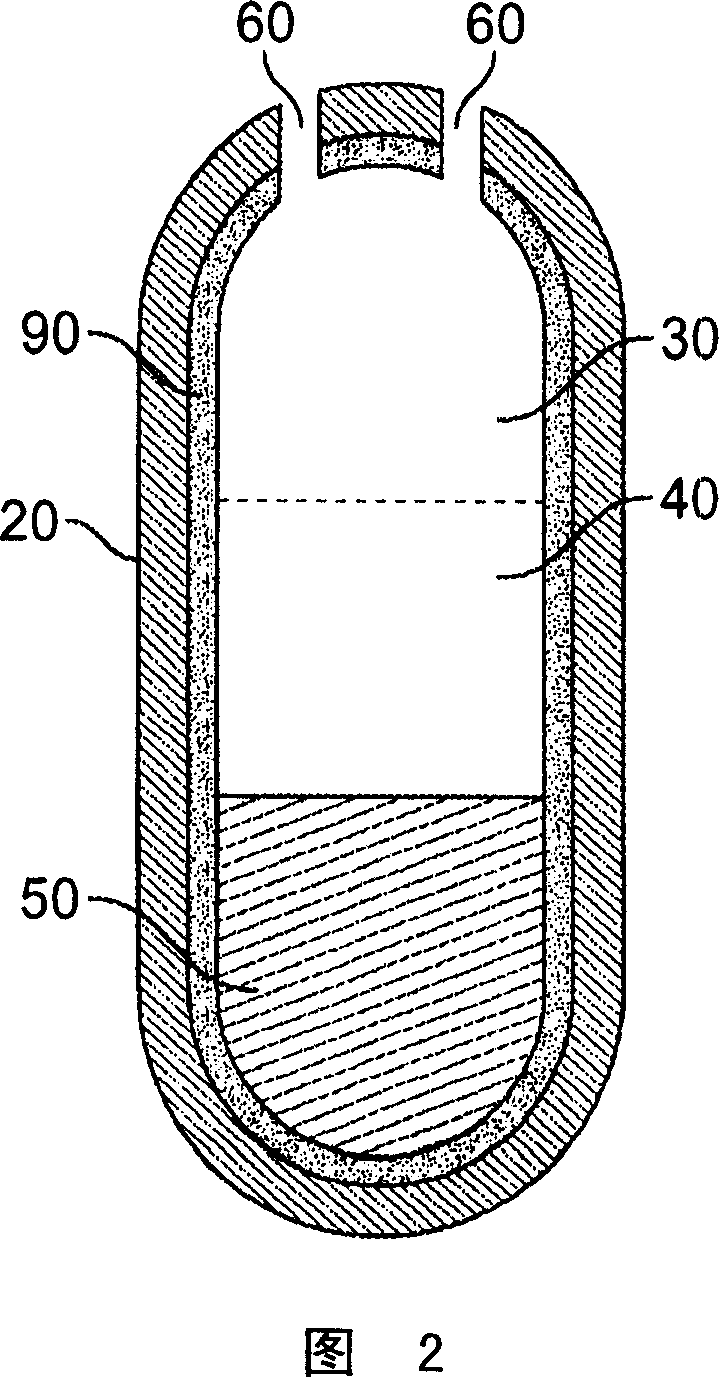
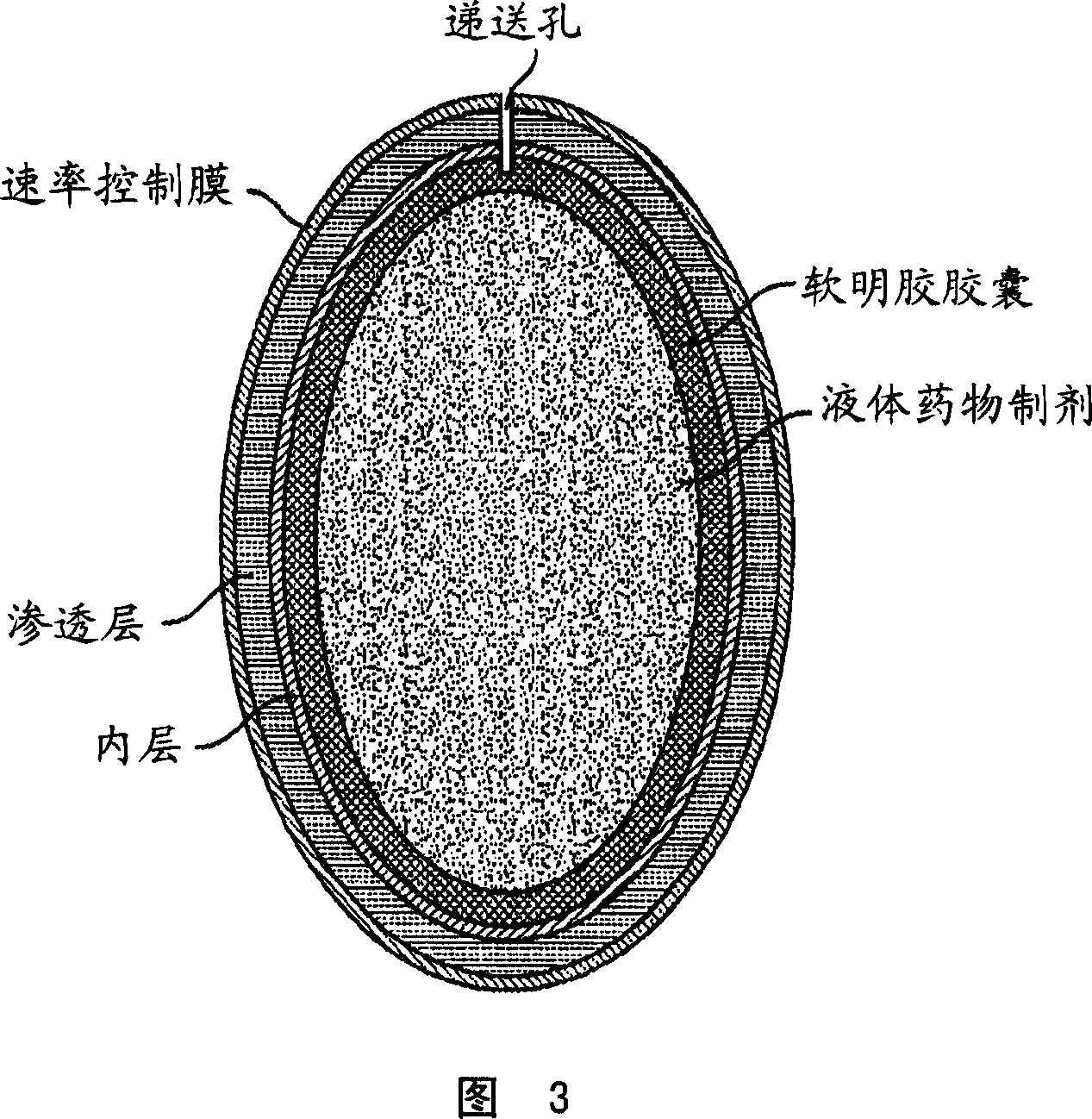
Dose dumping is a phenomenon of drug metabolism in which environmental factors can cause the premature and exaggerated release of a drug. This can greatly increase the concentration of a drug in the body and thereby produce adverse effects or even drug-induced toxicity.
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20090317355A1Reduced and limited dose-dumping effectReduce interactionBiocideNervous disorderVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Ethanol-resistant sustained release formulations
InactiveUS20070212414A1High drug safetyLower potentialBiocideNervous disorderSustained Release FormulationsDelivery system
The invention provides formulations that resist dose dumping in the presence of ethanol and methods of use thereof. The formulations can be used to prevent dose dumping, to increase safety of drugs, and to reduce abuse of drugs prone to such abuse. The formulations comprise at least one drug and a sustained release delivery system. In one embodiment, the drug is an opioid.
Owner:ENDO PHARMA INC
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20100172989A1Reduced and limited dose-dumping effectReduce interactionPowder deliveryBiocideVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG +1
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Pharmaceutical compositions and methods for mitigating risk of alcohol induced dose dumping or drug abuse
Abuse resistant polyglycol-based pharmaceutical compositions are disclosed. The composition contains one or more polyglycols and one or more active substances and it is resistant to crushing, melting and / or extraction. Moreover, such compositions have the same or lower solubility in ethanolic-aqueous medium, i.e. they are not subject to ethanol-induced dose dumping effect.
Owner:EGALET LTD
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
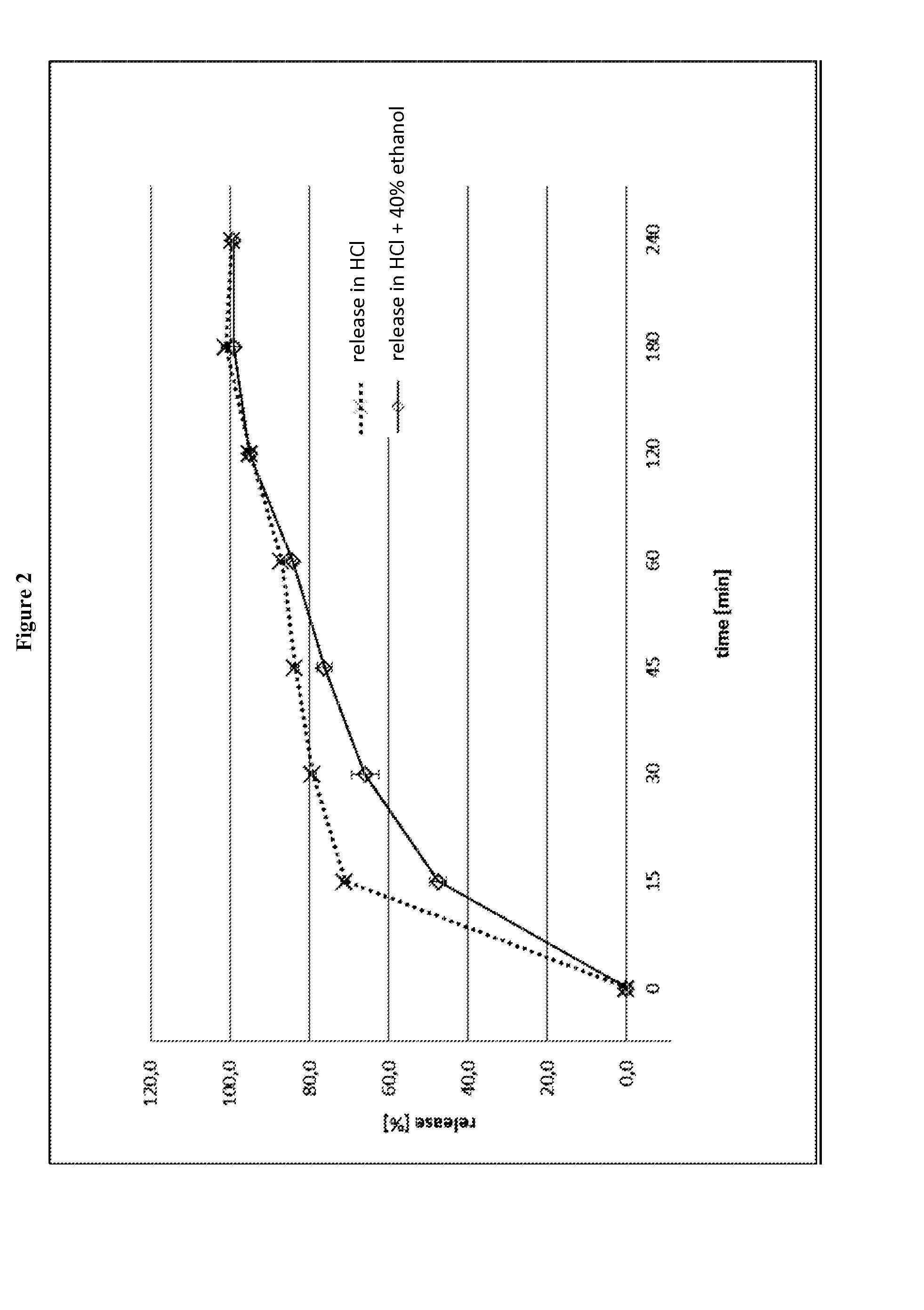
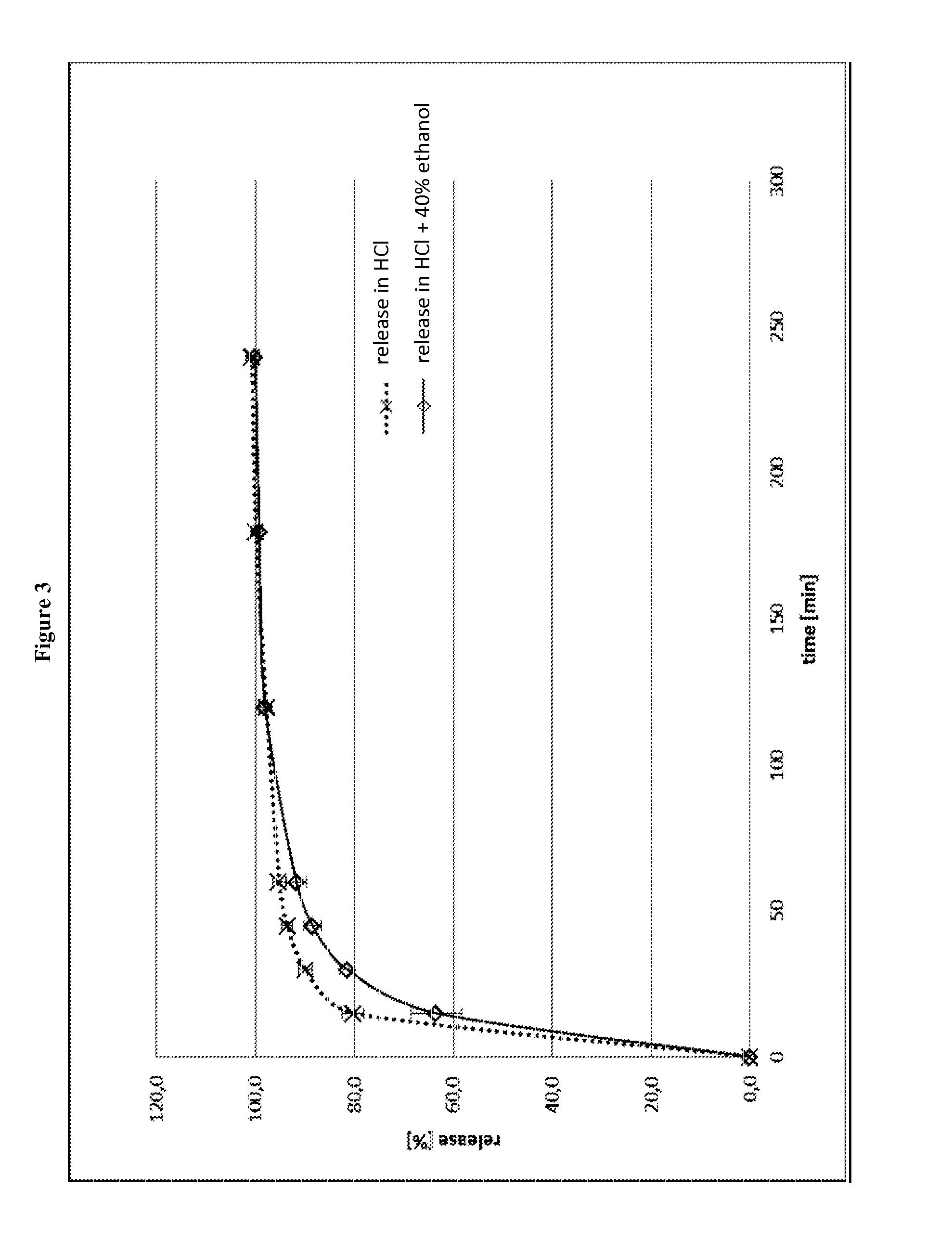
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Alcohol Resistant Pharmaceutical Formulations
InactiveUS20090155357A1Relieve painEffectively alleviatedOrganic active ingredientsNervous disorderAlcoholPharmaceutical formulation
The present invention provides alcohol resistant oral dosage pharmaceutical forms and methods of using such oral dosage forms to avoid dose dumping if the dosage form is taken together with alcohol.
Owner:ALPHARMA INC (US)
Robust sustained release formulations of oxymorphone
InactiveUS20080085305A1Avoid dose dumpingHigh drug safetyPowder deliveryOrganic chemistryOxymorphoneSustained Release Formulations
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Tamper-resistant dosage form containing ethylene-vinyl acetate polymer
The invention relates to a tamper-resistant, oral pharmaceutical dosage form comprising a pharmacologically active ingredient having psychotropic action and an ethylene-vinyl acetate (EVA) polymer which provides resistance against solvent extraction, resistance against grinding, and resistance against dose-dumping in aqueous ethanol.
Owner:GRUNENTHAL GMBH
Pharmaceutical compositions for reducing alcohol-induced dose dumping
A pharmaceutical composition is disclosed. The composition comprises a core comprising an active substance or a salt thereof; a separating layer comprising at least one sugar; and a functional layer comprising at least one pharmaceutically acceptable polymer, wherein the composition is resistant to dose dumping in presence of alcohol.
Owner:CADILA HEALTHCARE LTD
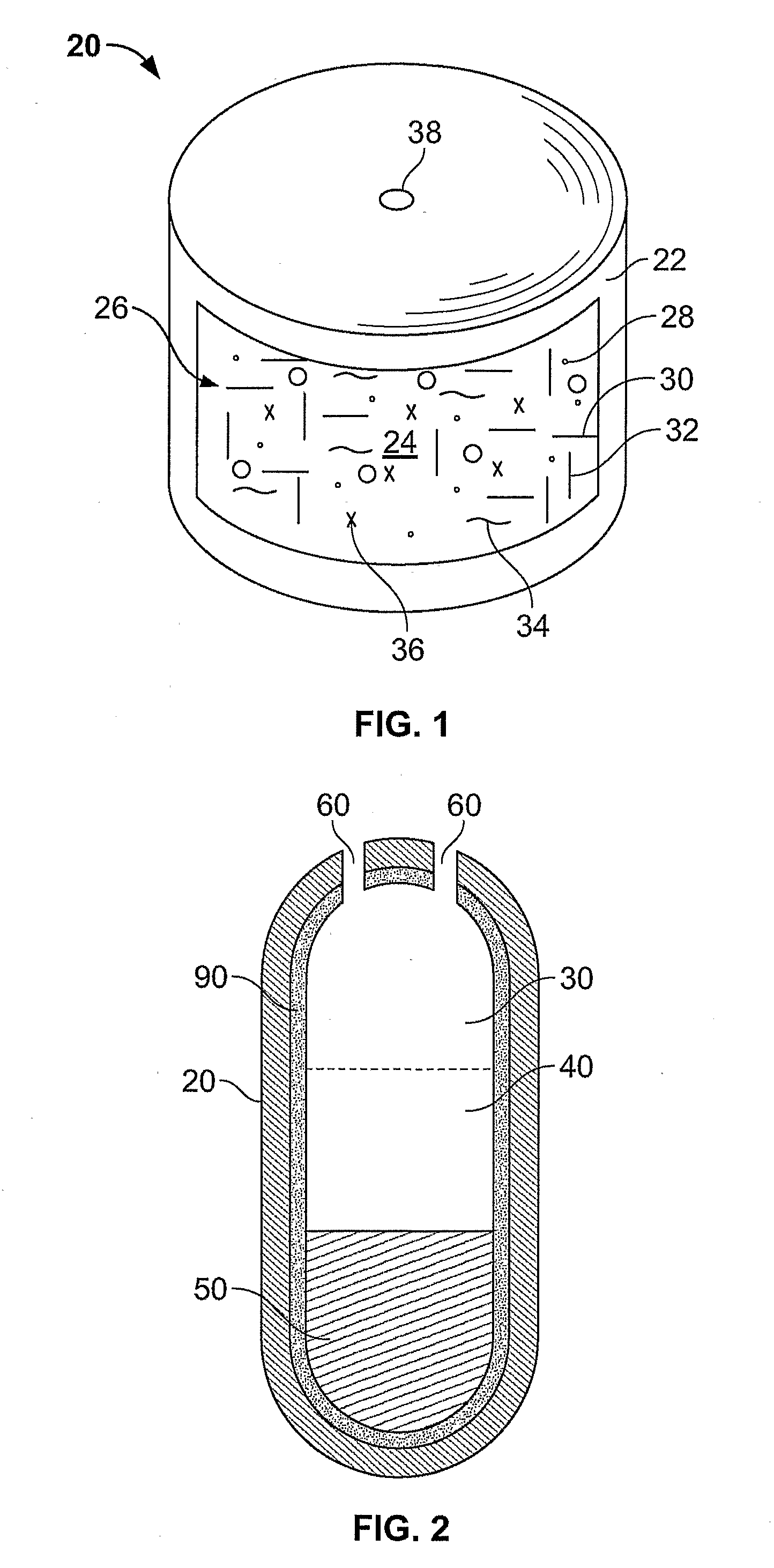
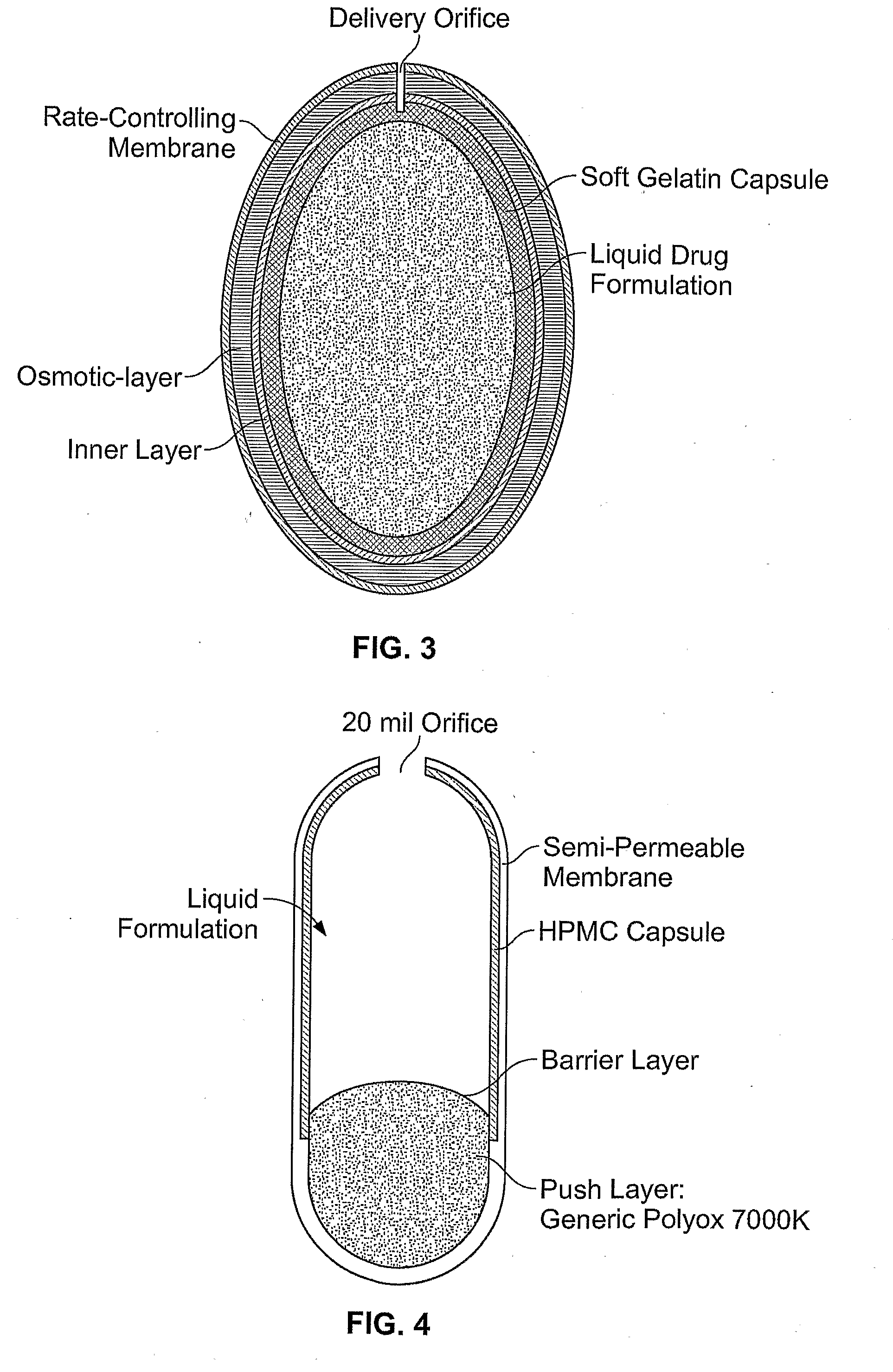
Methods of Reducing Alcohol-Induced Dose Dumping for Opioid Sustained Release Oral Dosage Forms
Disclosed are methods of sustained release administration of opioids, including but not limited to hydromorphone and oxycodone, that exhibit improved properties with respect to co-ingestion with aqueous alcohol.
Owner:ALZA CORP
Ilaprazole enteric-coated tablets and preparation method thereof
InactiveCN102525990AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemActive agentSurface-active agents
The invention provides ilaprazole enteric-coated tablets and a preparation method thereof. Each enteric-coated tablet contains an enteric-coated pellet and pharmaceutically acceptable tablet excipients, wherein the enteric-coated pellet contains a pellet layer, a medicine loading layer, an isolation layer and an enteric coating layer; and the medicine loading layer contains ilaprazole or pharmaceutically acceptable salt thereof and a stabilizer. The enteric-coated pellet tablets prepared from the ilaprazole have good acid resistance; barrier substances such as an antiacid, a surfactant, an organic solvent, a hydrophobic substance and the like are not contained in the prescription, so that the enteric-coated pellet tablets have health benefits and are safe; the preparation method is easy to operate, the organic solvent is not used, and an active substance is quickly and stably released; and in addition, the pellets in the tablets can be widely and uniformly distributed in an intestinal tract after being taken, the dose dumping is dispersed, and the distribution area of a medicine on the surface of the intestinal tract is increased, so the irritation of the medicine to the intestinal tract can be reduced or eliminated, and the bioavailability of the medicine can be improved.
Owner:LIVZON PHARM GRP INC
Alcohol-resistant drug formulations
ActiveUS20200330393A1Eliminate side effectsNervous disorderGranular deliverySleep arousalParacetamolum
The invention relates to modified release oral formulations of therapeutic agents, including gamma hydroxybutyrate (GHB), paracetamol, codeine or oxycodone, which are resistant to alcohol induced dose dumping. Provided are formulations that have improved resistance to rapid release of the active ingredient in the presence of increasing amounts of alcohol. Also provided are formulations that can reduce or prevent the release of the active ingredient following exposure to alcohol-containing media. The invention also relates to methods of making the formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Tamper resistant and dose-dumping resistant pharmaceutical dosage form
ActiveUS20130280338A1Facilitated releaseOrganic active ingredientsBiocideAqueous ethanolProlonged release
A tamper-resistant pharmaceutical dosage form comprising a pharmacologically active ingredient embedded in a prolonged release matrix, which comprises a prolonged release matrix material selected from the group consisting of nonionic acrylic polymers and waxy materials and which provides prolonged release of the pharmacologically active ingredient, resistance against solvent extraction, resistance against grinding, and resistance against dose-dumping in aqueous ethanol.
Owner:GRUNENTHAL GMBH
Oral controlled release tablet
A method of reducing the risk of alcohol-induced dose-dumping of a therapeutically active ingredient comprising administering to human subjects who have ingested alcohol an oral controlled release tablet; said tablet comprising:a core comprisingan upper compressed layer comprising a swelling agent, anda lower compressed layer comprising at least one therapeutically active ingredient, and pharmaceutically acceptable excipient wherein at least one excipient is a release rate controlling excipient and wherein the percent by weight of excipients that are soluble in alcohol does not exceed 35% by weight of the layer and;a coating surrounding the said core, the coating comprising a polymer insoluble in an aqueous medium comprising from 0% v / v to 40% v / v of alcohol, whereby upon contact with aqueous gastrointestinal fluids, the upper compressed layer swells to cause removal of the coating from the upper surface of the upper compressed layer and then said upper layer disintegrates allowing the release of the active ingredient from the defined surface area of the upper surface of said lower compressed layer with the coating covering its bottom and side surfaces.
Owner:SUN PHARMA INDS
Multiparticles safeguarded against ethanolic dose-dumping
The invention relates to an oral pharmaceutical dosage form providing resistance against dose dumping in aqueous ethanol and comprising a pharmacologically active ingredient embedded in a matrix material,wherein the matrix material comprises an alkyl cellulose and a heteropolysaccharide; andwherein the relative weight ratio of heteropolysaccharide to alkyl cellulose is within the range of from 1:20 to 20:1; andwherein the total content of alkyl cellulose and heteropolysaccharide is at least 35 wt.-%, relative to the total weight of the dosage form. A process of producing the dosage form and methods of using the dosage form, for example to treat pain, are also disclosed.
Owner:GRUNENTHAL GMBH
Opioid salts and formulations exhibiting anti-abuse and anti-dose dumping properties
Owner:PISGAH LAB
DMF (dimethyl fumarate) enteric-coated micropellet and preparation method thereof
ActiveCN104352441AReduce gastrointestinal side effectsAvoid sublimationOrganic active ingredientsPharmaceutical non-active ingredientsMedicineExtrusion spheronization
The invention relates to a DMF (dimethyl fumarate) enteric-coated micropellet and a preparation method thereof. The DMF enteric-coated micropellet comprises a pellet core and a double-layer enteric coating, wherein the pellet core is coated with the double-layer enteric coating. The enteric-coated micropellet is prepared by the following steps: the pellet core is prepared with the extrusion-spheronization method; and the pill-containing pellet core is coated with the double-layer enteric coating to form the enteric-coated micropellet. The obtained DMF enteric-coated micropellet is simple in preparation technology, complete in drug release and good in reproducibility, and external dose dumping risk is reduced.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Opioid and attention deficit hyperactivity disorder medications possessing abuse deterrent and anti-dose dumping safety features
ActiveUS10183001B1Pharmaceutical delivery mechanismHeterocyclic compound active ingredientsDrug productAbuse deterrent
An abuse deterrent drug product is provided wherein the drug product comprises a matrix and a drug substance in the matrix wherein the drug substance is defined as a 1:1 salt of a pharmaceutically active compound and BNDO.
Owner:PISGAH LAB
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20110104266A1Avoid and limit doseTherapy is simplePowder deliveryOrganic active ingredientsOral medicationPharmaceutical medicine
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms.The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium.The form comprises an agent 13, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution.
Owner:FLAMEL TECHNOLOGIES
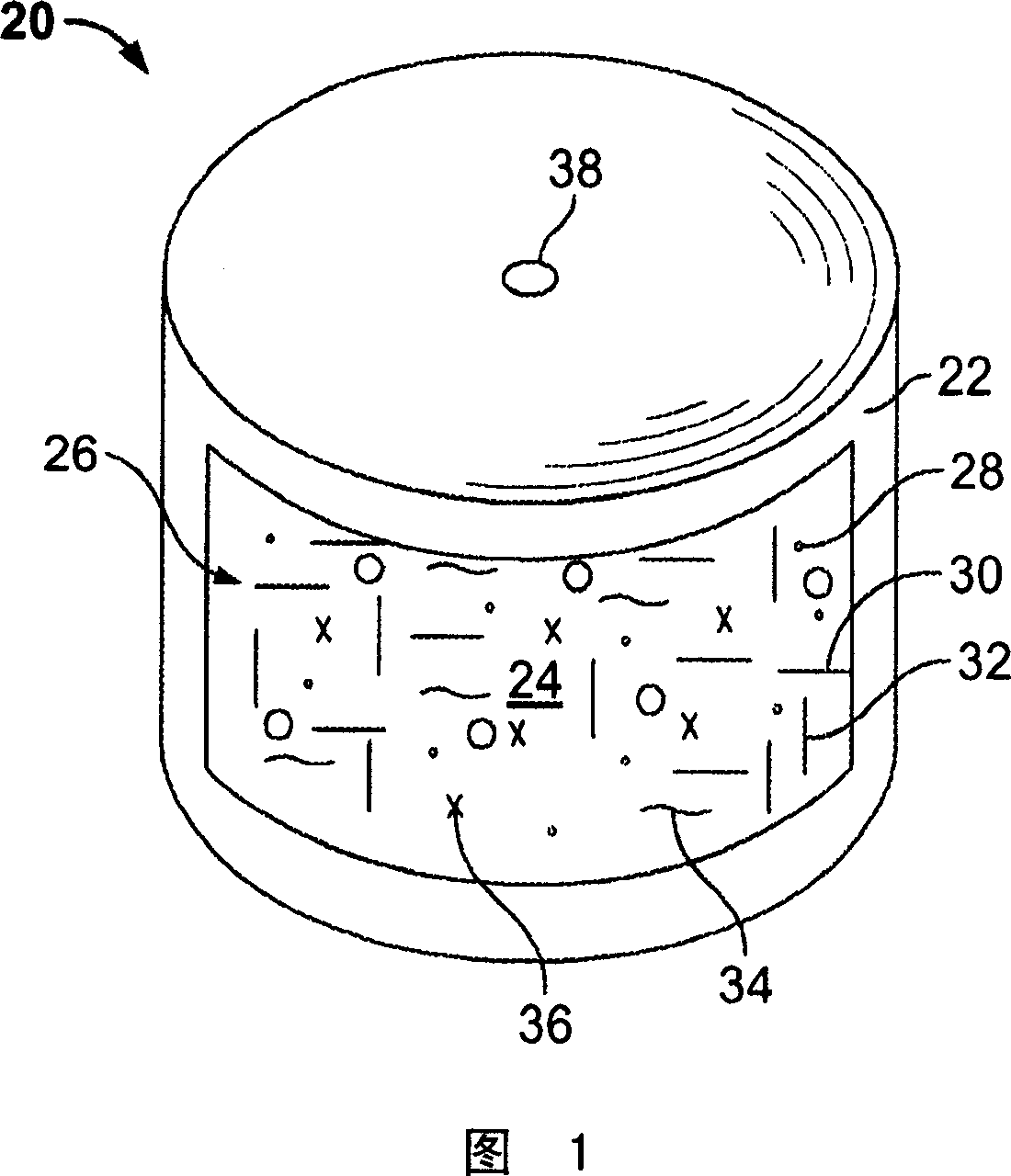
Slow-release medicine carrier
InactiveCN101983723AGood drug releaseGood drug release propertiesSuppositories deliveryMacromolecular non-active ingredientsIrritationIn vivo
The invention discloses a slow-release medicine carrier with improved performance, which includes (a) fatty glyceride whose melting point in vivo and vitro is more than 37 DEG C; (b) additive particles which are soluble in water, has low viscosity and no irritation; (c) an acidic (or alkalic) polymer with water swelling ability and water insolubility; (d ) an alkalic (or acidic) surfactant; and (e) a medicament. The medicament carrier has better medicament-release characteristic, high salt poisoning resistance, low irritation to mucous membrane, better biological compatibility and better medicament-release homogeneity and better relieves or solves the problem of end release; and meanwhile it is not easy to generate medicament safety problems such as burst release or dose dumping of medicament.
Owner:钟术光
Robust sustained release formulations of oxymorphone and methods of use thereof
InactiveUS20080085303A1Avoid dose dumpingHigh drug safetyBiocidePowder deliveryOxymorphoneSustained Release Formulations
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Sustained-release solid preparation for oral use
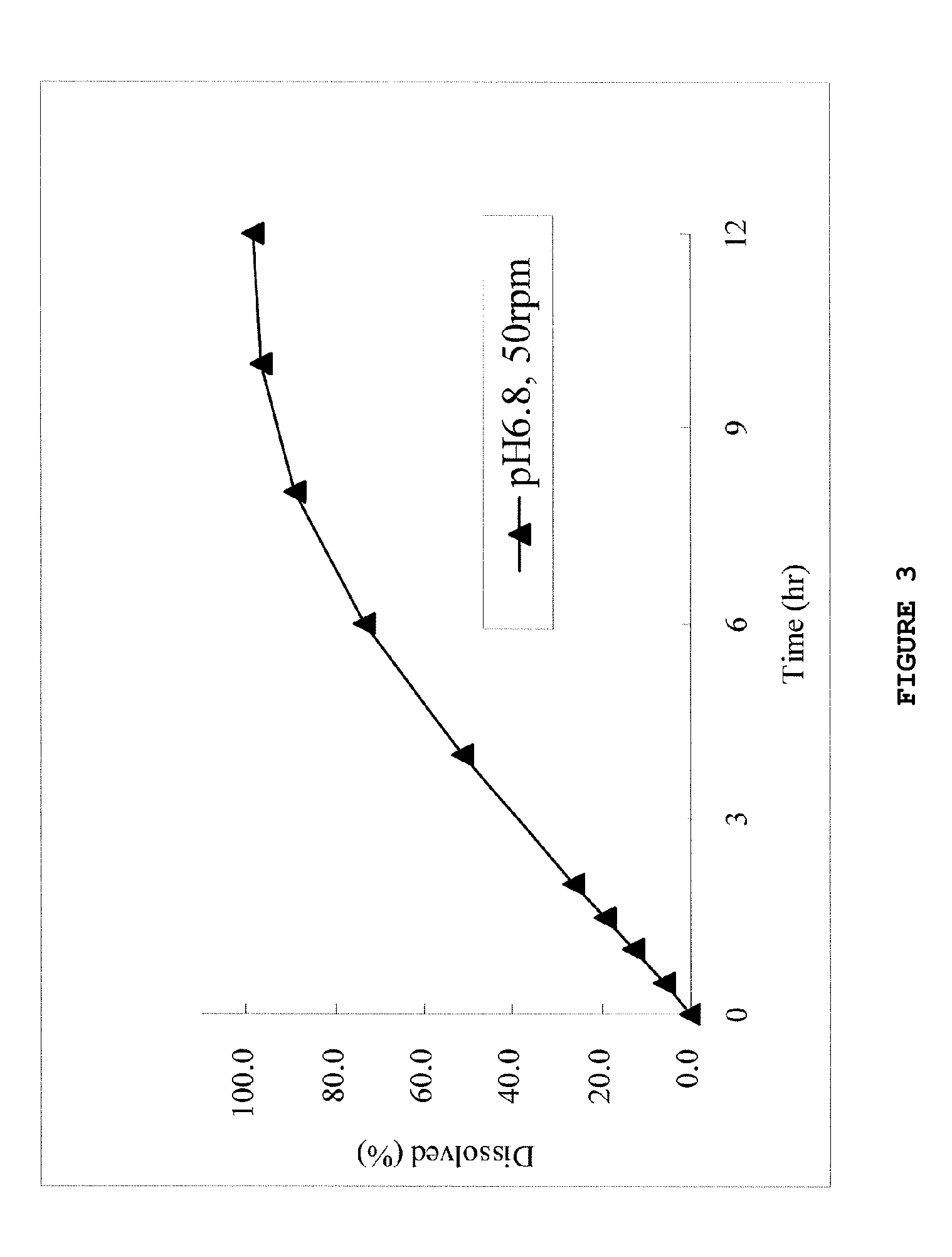
InactiveUS20130012535A1High strengthAvoid dose dumpingPowder deliveryBiocideSustained release pelletsLower Gastrointestinal Tract
It is intended to avoid dose dumping of a drug and improve the dissolution properties of the drug in the lower gastrointestinal tract, and thereby provide a sustained-release pellet preparation for oral administration that reliably exhibits its main pharmacological effect when orally administered once or twice a day. The present invention provides a sustained-release preparation obtained by mixing of (A) a pharmacologically active drug, (B) hydroxypropyl methylcellulose acetate succinate, (C) a plasticizer, and (D) polyethylene glycol followed by extrusion granulation.
Owner:DAIICHI SANKYO CO LTD
Drug carrier capable of realizing sustained release of drug
InactiveCN102000339AGood drug releaseGood drug release propertiesSuppositories deliveryMacromolecular non-active ingredientsHigh resistanceIrritation
The invention discloses a drug carrier capable of realizing sustained release of a drug, which improves the performances and contains (a) an aliphatic additive, wherein the in vivo melting point and the in vitro melting point are higher than the temperature of 37 DEG C; (b) water-soluble additive particles with low viscosity and no irritation; (c) a water-insoluble acidic (or alkaline) polymer with water swelling property; (d) an alkaline (or acidic) surfactant; and (e) the drug. The drug carrier has better drug release property, higher resistance to salt poisoning, lower mucosa irritation, better biocompatibility and better drug release uniformity, can better ease or solve the problem of end release, and is difficult to cause initial burst release of the drug or dose dumping and other drug safety problems.
Owner:钟术光
Abuse deterrent and Anti-dose dumping pharmaceutical salts useful for the treatment of attention deficit/hyperactivity disorder
A pharmaceutical composition comprising a drug substance consisting essentially of a pharmaceutically acceptable organic acid addition salt of an amine containing pharmaceutically active compound wherein the amine containing pharmaceutical active compound is selected from the group consisting of racemic or single isomer ritalinic acid or phenethylamine derivatives and the drug substance has a physical form selected from amorphous and polymorphic.
Owner:PISGAH LAB
Abuse and alcohol resistant drug composition
ActiveUS20140010860A1Reduced drug abuse potentialNo abuse potentialPowder deliveryOrganic active ingredientsSodium BentoniteNasal route
A liquid or semi-solid matrix or magma or paste which is non-newtonian, thixotropic and pseudoplastic and composed of one or more controlled release agent, and / or one or more clays such as bentonite and / or one or more fillers in a non aqueous vehicle, and optionally materials selected from disintegrants, humectants, surfactants and stabilizers. The composition and physicochemical properties makes it harder or prevents dose dumping of narcotic analgesics in the presence of alcohol and harder to abuse opiod agonists or narcotic analgesics and discourages drug abuse via crushing, milling or grinding the dosage form to powder or heating the dosage form to vapour and snorting or inhalation by the nasal route or dissolving to abuse via the parenteral route.
Owner:INTELLIPHARMACEUTICS
A drug carrier for slow-release medicine
InactiveCN102258785AGood drug releaseGood drug release propertiesSuppositories deliveryPharmaceutical non-active ingredientsIrritationWater insoluble
The invention discloses a slow-release drug carrier with improved performance. The drug carrier contains (a) an aliphatic additive in vivo-in vitro fusing points of which is higher than 37 DEG C, (b) low-viscosity water-soluble nonirritant additive particles, (c) a water-swelling and water-insoluble acidic (or alkaline) polymer, (d) an alkaline (or acidic) surfactant, and (e) a medicament. The drug carrier has the advantages of better drug release character, higher 'salt poisoning' resistance effect, lower mucosal irritation, better biocompatibility and better drug release uniformity, thus better relieving or solving 'terminal release' problem and avoiding medication safety problems such as burst drug release or dose dumping and the like.
Owner:钟术光
Sustained-release solid preparation for oral use
InactiveUS20130005763A1Increase ratingsAvoid dose dumpingBiocidePill deliveryCarmellose SodiumLower Gastrointestinal Tract
It is intended to avoid dose dumping of a drug and improve the dissolution properties of a drug in the lower gastrointestinal tract, and thereby provide a sustained-release solid preparation for oral administration that reliably exhibits its main pharmacological effect when orally administered once or twice a day. The present invention provides a sustained-release solid preparation containing (A) a pharmacologically active drug, (B) carboxyvinyl polymer, (C) povidone, and (D) carmellose sodium, xanthan gum, or sodium carboxymethyl starch.
Owner:DAIICHI SANKYO CO LTD
Sustained-release solid preparation for oral use
InactiveUS20130004550A1Favorable tablet strengthAvoid dose dumpingPowder deliveryBiocideAcetic acidLower Gastrointestinal Tract
It is intended to avoid dose dumping of a drug and improve the dissolution properties of the drug in the lower gastrointestinal tract, and thereby provide a sustained-release matrix preparation for oral administration that reliably exhibits its main pharmacological effect when orally administered once or twice a day. The present invention provides a sustained-release preparation obtained by mixing of (A) a pharmacologically active drug, (B) hydroxypropyl methylcellulose acetate succinate having a median size (D50) of 40 μm or smaller, (C) a cellulose derivative, and (D) a saccharide or a nonionic water-soluble polymer followed by molding.
Owner:DAIICHI SANKYO CO LTD
Methods of reducing alcohol-induced dose dumping for opioid sustained release oral dosage forms
Disclosed are methods of sustained release administration of opioids, including but not limited to hydromorphone and oxycodone, that exhibit improved properties with respect to co-ingestion with aqueous alcohol.
Owner:ALZA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com