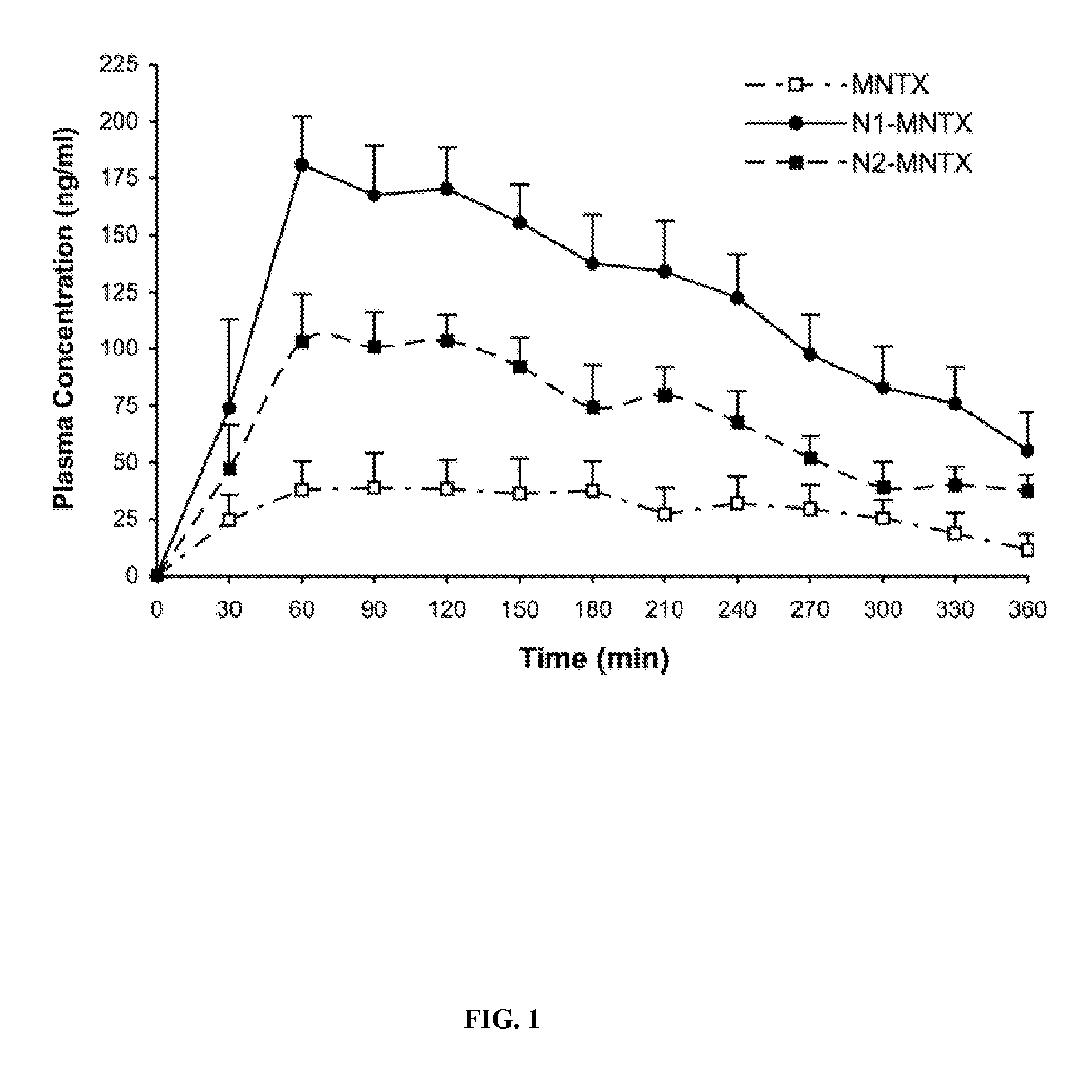
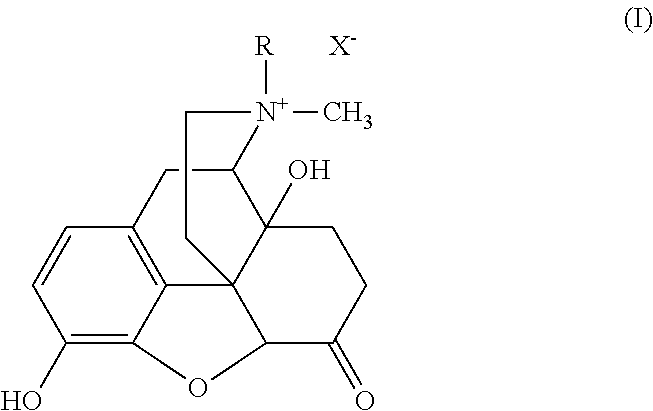
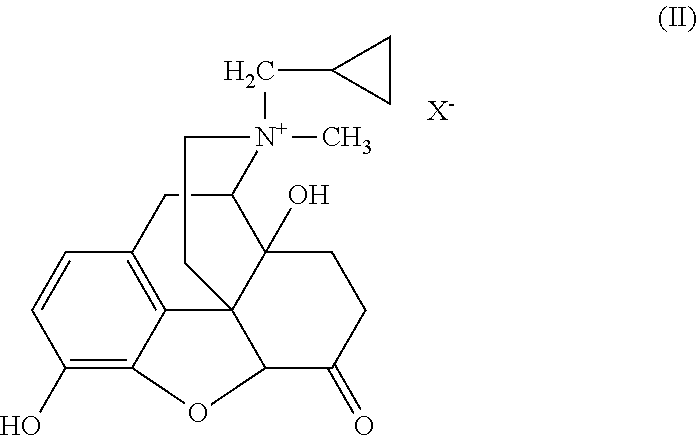
Particles containing an opioid receptor antagonist and methods of use
a technology of opioid receptor and opioid receptor, applied in the field of opioid receptor antagonists and drug delivery, can solve the problems of limited efficacy of common treatments of bulking agents and laxatives, affecting so as to improve the absorption of opioid receptor antagonists. , the effect of reducing the dose required
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Particles Comprising Methylnaltrexone
[0138]A procedure developed by the Alonso lab from the School of Pharmacy, University of Santiago de Compostela, Spain was employed (Calvo et al., 1997; Fernandez-Urrusuno et al., 1999).
[0139]Methylnaltrexone (MNTX) (Mallinckrodt Chemicals, St. Louis, Mo.) was dissolved in water and then incorporated in an aqueous pentasodium tripolyphosphate (TPP) solution. Under high-speed magnetic stirring of an aqueous chitosan solution, the MNTX-containing TPP solution was slowly added into the chitosan solution. Nanoparticles containing MNTX were then formed. The final ratio of chitosan:TPP:MNTX was approximately 5 / 1.8 / 3.2 (w / w / w). MNTX nanoparticles were collected by centrifugation, supernatants were discarded and the remaining nanoparticles were lyophilized.
example 2
Preparation of Enterically Coated Particles Comprising Methylnaltrexone
[0140]Enterically coated MNTX nanoparticles were prepared by encapsulating the nanoparticles of Example 1 with a Eudagrit® L100 and Myvacet® 9-45 mixture. See, e.g., U.S. Pat. No. 6,608,075 and Yuan et al., 2000, each of which is incorporated herein by reference in its entirety. The final substance was the 30-80 mesh fraction which was 60% MNTX nanoparticles by weight. It was shown to decrease release of the drug at gastric pH by 90% based on the methods of the United States Pharmacopoeia / National Formulary (The United States Pharmacopeia, 1995). See also U.S. Pat. No. 6,608,075 and Yuan et al., 2000.
example 3
Preparation of a Heteroparticulate Particle Comprising Methylnaltrexone
[0141]Methodology as described by Beck et al., 2004 was followed. To prepare the outer particles, a lipophilic solution consisting of Epikuron 170® (0.1532 g), a polymer (poly(caprolactone) (PCL) (MW=60,000 g / mol) or Eudragit® 5100) (1.0 g) and acetone (267.0 ml) was used. This organic phase was added to an aqueous solution (533.0 ml) containing Tween 80® (0.1532 g) under moderate magnetic stirring. The solution was concentrated by evaporation under reduced pressure, and then the final volume was adjusted to 100 ml using acetone, corresponding to a polymer concentration of 10 mg / ml.
[0142]To prepare the inner particle, an MNTX solution (17 mM, 50 mL) was added to Aerosil® 200 (1.50 g). The mixture was fed into a mini-spray-dryer to produce particles having an MNTX core (feed rate: 3.0 ml / min; air flow rate: 500 NL / hr; atomizing air pressure: 200 kPa; inlet temperature: 170±4° C.; outlet temperature: 110±4° C.; noz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com