Process for the preparation of protected L-alanine derivatives
a technology of l-alanine and derivatives, which is applied in the preparation of sulfonic acid esters, carbamic acid derivatives, organic chemistry, etc., can solve the problems of reducing the desired binding activity of n-substitutions on carboxamides, and the likelihood of inherent instability of instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-tert-Butoxycarbonylamino-3-(4-carbamoyl-2,6-dimethyl-phenyl)-propionic acid methyl ester
[0108]
STEP A
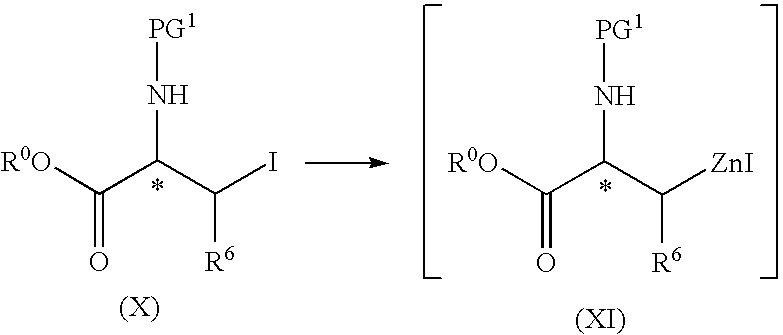
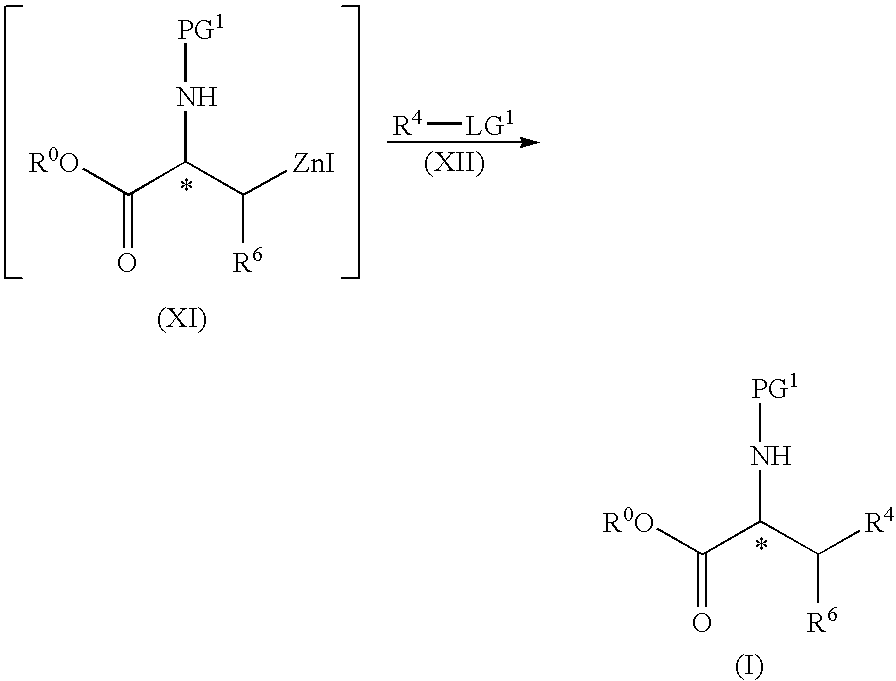
[0109]Dry DMAc (300 mL), 2-Me-THF (150 mL), I2 (25.4 g, 0.10 mol) and zinc powder (294.3 g, 4.5 mol), were added under nitrogen to a 3 L four-necked round bottom flask equipped with an addition funnel, mechanical stirrer, heating mantel, condenser and thermocouple. The resulting slurry was stirred until the red color of I2 disappeared (about 2 minutes). During the addition, a temperature increase was observed (from 23° C. to 43° C.). The resulting mixture was cooled down using an ice / NaCl bath to about −5° C. to −2° C. While at this temperature, a solution of Boc-6-iodo-alanine-OCH3 (also known as 2-tert-butoxycarbonylamino-3-iodo-propionic acid methyl ester, 658.3 g, 2.0 mol) in a mixture of DMAc (250 mL) and 2-Me-THF (500 mL) was added slowly over a period of 2 hours. The temperature of the resulting mixture was maintained below 10° C. and the mixture aged for a per...
example 2
Preparation of (S)-2-tert-Butoxycarbonylamino-3-(4-cyano-2,6-dimethyl-phenyl)-propionic acid methyl ester
[0112]
[0113]A 50 mL three-necked round bottom flask equipped with an addition funnel, magnetic stirrer, heating mantel, and thermocouple was charged under nitrogen dry DMAc (2 mL), I2 (38.1 mg, 0.15 mmol) and zinc powder activated (washed with 10% HCl, rinsed with H2O and acetone) (393 mg, 6 mmol). The resulting mixture was stirred at 23° C. until the red color of I2 disappeared (2 minutes). A solution of Boc-β-iodo-L-alanine methyl ester (1 g, 3 mmol) in DMAc (2 mL) was added slowly, (temperature change from 21° C. to 29° C.) and the resulting mixture was stirred at 80° C. for 0.5-1 hour, then co cooled to 35° C. To the resulting mixture were added, successively, 4-bromo-3,5-dimethyl-benzonitrile (315 mg, 1.5 mmol) in DMAc (6 mL), P(o-tol)3 (36.5 mg, 0.12 mmol) and Pd2(dba)3 (55 mg, 0.06 mmol). The resulting mixture was heated to 70° C., with stirring for 1 hour, then cooled to ...
example 3
Preparation of (S)-2-tert-Butoxycarbonylamino-3-(4-cyano-2,6-dimethyl-phenyl)-propionic acid methyl ester
[0114]
STEP A: Boc-β-iodo-Alanine methyl ester
[0115]A 2 L four-necked round-bottomed flask equipped with a nitrogen inlet, a mechanical stirrer, an addition funnel and a thermocouple was charged with anhydrous DMAc (500 mL) and iodine (16.8 g, 0.06 mol) to yield a red solution. To the stirred solution was then added zinc powder (143.9 g, 2.2 mol). The red color of the resulting mixture was observed to disappear in about 2 minutes, and an exotherm (22° C. to about 36° C.) was observed. The resulting mixture was cooled to −8° C. and then a solution of N-(tert-butoxycarbonyl)-3-iodo-L-alanine methyl ester (658 g, 2.0 mol) in anhydrous DMAc (500 mL) was added slowly over about 2 hours, maintaining the mixture temperature at below about 10° C., without stirring. The resulting cooled mixture was used in the next step without further manipulation.
STEP B: (S)-2-tert-Butoxycarbonylamino-3-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com