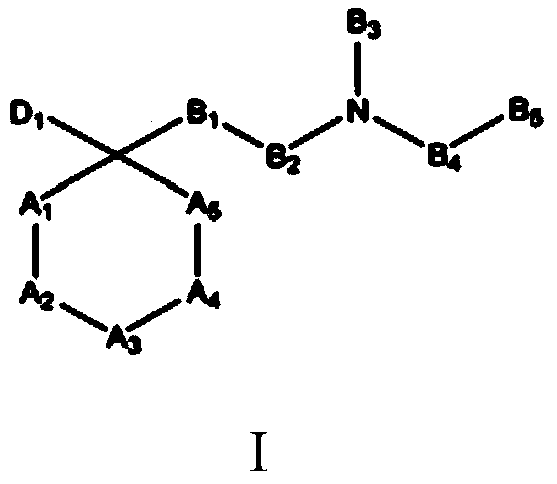
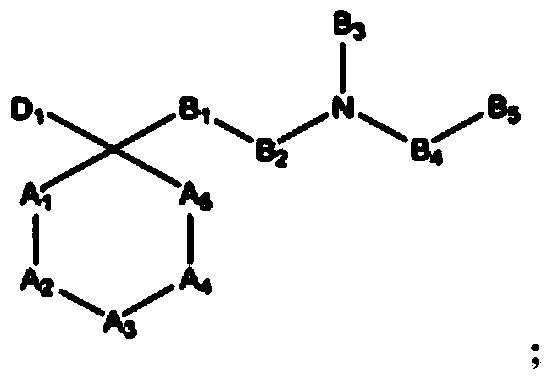
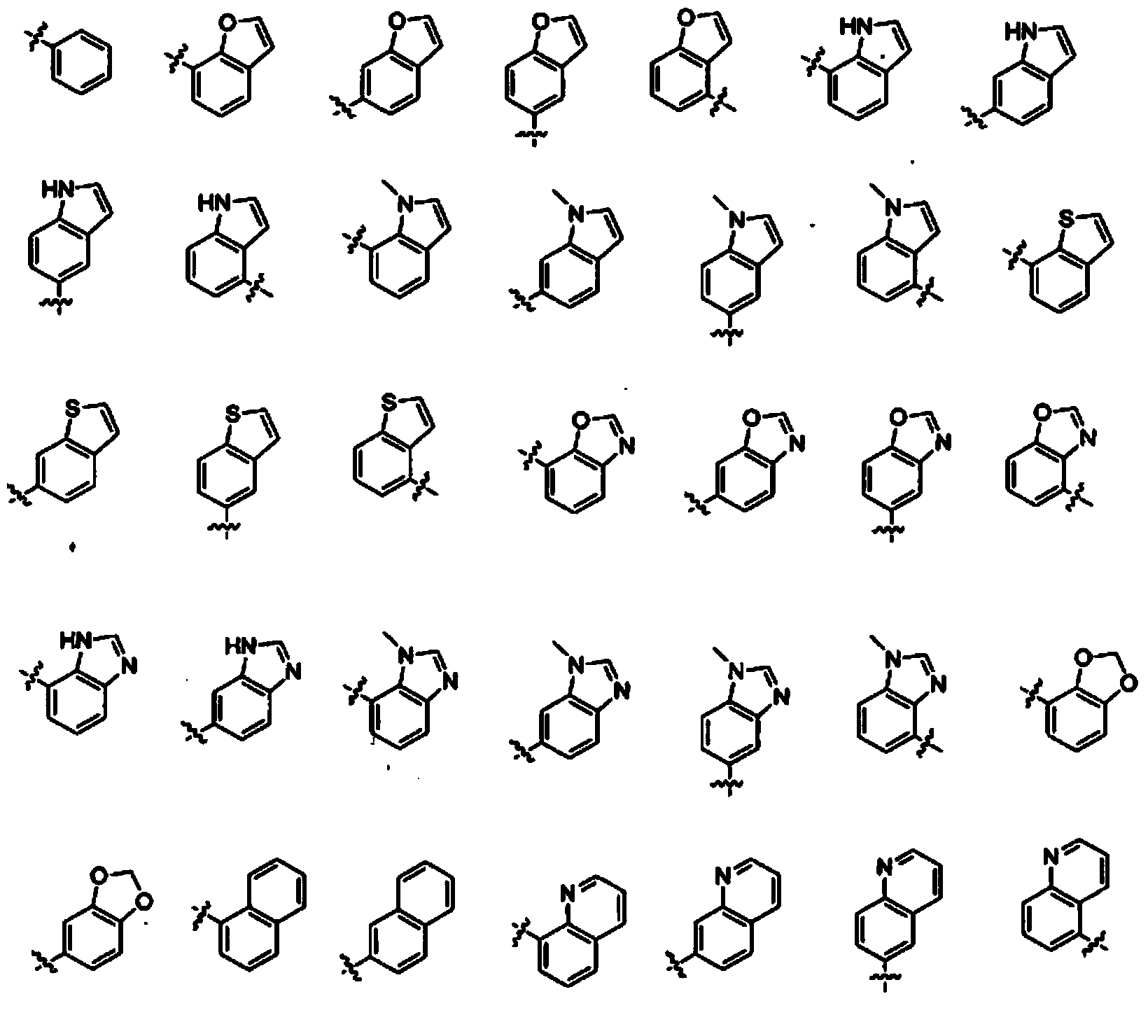
Opioid receptor ligands and methods of using and making same
A compound and free technology, applied in botany equipment and methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as vomiting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] Intermediate 1: Methyl 2-cyano-2-(oxan-4-ylidene)acetate (2-cyano-2-(oxan-4-ylidene)methyl acetate) Fill tetrahydro-4H-pyran-4-one (4.61ml, 50mmol), methyl cyanoacetate (5.3ml, 60mmol), ammonium acetate (1g, 13mmol) in the 50ml round bottom flask of distillation apparatus and condenser, Acetic acid (0.57ml, 10mmol) and benzene (30ml). The mixture was refluxed until no more water was collected in the Dean-Stark (2 hours), cooled, benzene (30ml) added and the organic layer washed with water (50ml). use CH 2 Cl 2 (3x50ml) to extract the aqueous layer. The combined organic layers were washed with saturated NaHCO 3 (100ml), washed with brine (100ml), dried (MgSO 4 ), filtered and concentrated. Via normal phase SiO 2 Chromatographic (10 to 60% EtOAc / hexanes) purification to give methyl 2-cyano-2-(oxan-4-ylidene)acetate (6.30 g, 70%, found m / z: 181.1 [ M+H] + ), as a colorless oil.
[0249] Intermediate 2: Methyl 2-cyano-2-[4-(4-fluorophenyl)oxan-4-yl]acetate
...
Embodiment 2
[0255] Example 2: Benzyl({2-[4-(4-fluorophenyl)oxan-4-yl]ethyl})amine (compound 8)
[0256] At room temperature, to anhydrous CH 2 Cl 2 2-[4-(4-fluorophenyl)oxan-4-yl]ethan-1-amine (250mg, 1.12mmol) and Na in (5ml) 2 SO 4 (159mg, 1.12mmol) was added benzaldehyde (0.17ml, 1.68mmol). The reaction was stirred overnight. The reaction mixture was filtered and concentrated. The residue was dissolved in 5 ml MeOH at 0 °C and NaBH was added in one portion 4 (51 mg, 1.34 mmol). The reaction was stirred at 0 °C for 1 hour. The solution was then treated with H 2 O (10ml), quenched with CH 2 Cl 2 (3x20ml) was extracted, washed with brine (10ml) and dissolved in Na 2 SO 4 Dry on top. Via normal phase SiO 2 Chromatography (0 to 10% MeOH / CH 2 Cl 2 ) was purified to give benzyl({2-[4-(4-fluorophenyl)oxan-4-yl]ethyl})amine (200 mg, 60%, found m / z: 314.2 [M+ H] + ), as a colorless oil.
[0257] Intermediate 5: 2,2-Dimethyl-4-(4-methylphenyl)oxan-4-ol
[0258] in N 2 n-B...
Embodiment 3
[0265] Example 3:{2-[2,2-Dimethyl-4-(4-methylphenyl)oxan-4-yl]ethyl}[(3-methylphenyl)methyl]amine (compound 32 )
[0266] will be in CH 2 Cl 2 2-[2,2-Dimethyl-4-(4-methylphenyl)oxan-4-yl]acetaldehyde (61.6 mg, 0.25 mmol), 3-methylbenzyl A mixture of amine (63 µl, 0.5 mmol) and acetic acid (50 µl, 8.6 mmol) was stirred at room temperature for 1 hour, then sodium triacetoxyborohydride (106 mg, 0.50 mmol) was added. The resulting mixture was stirred at room temperature for 18 hours. The mixture was concentrated and dissolved in MeOH, and purified by HPLC to give {2-[2,2-dimethyl-4-(4-methylphenyl)oxan-4-yl]ethyl}[( 3-Methylphenyl)methyl]amine (35 mg, 40%, found m / z: 352.3 [M+H] + ), as a white solid.
[0267] Intermediate 8: Methyl 2-cyano-2-[(9Z)-6-oxaspiro[4.5]decane-9-ylidene]acetate
[0268] A 100ml round-bottomed flask equipped with a Dean-Stark distillation apparatus and a condenser was charged with 6-oxaspiro[4.5]decane-9-one (6g, 39mmol, according to Hanschke, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com