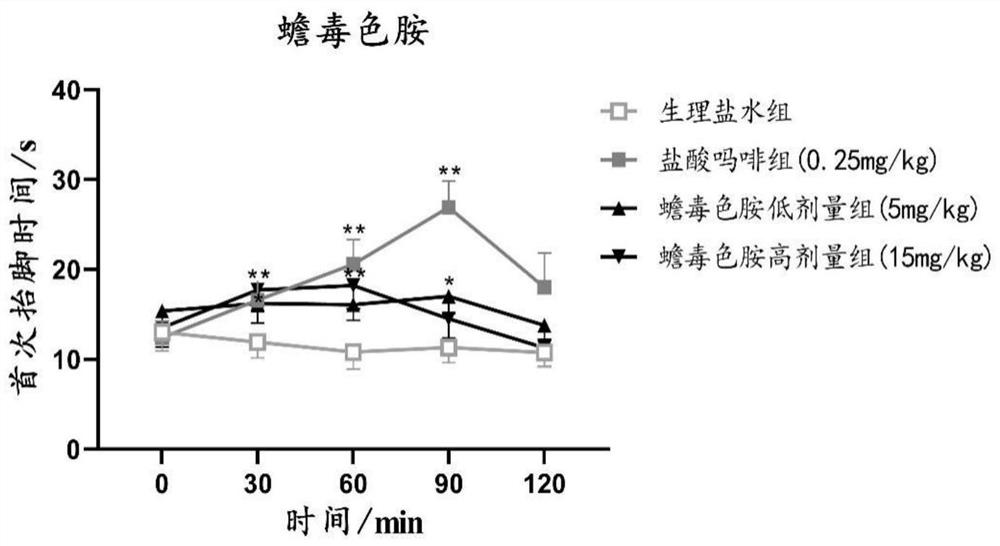
A kind of synthetic method of bufotryptamine and its quaternary ammonium salt and its application in the preparation of analgesic and anti-inflammatory drugs
A technology of bufotryptamine and quaternary ammonium salt, which is applied in analgesia, synthesis of bufotryptamine and its quaternary ammonium salt, and application fields of anti-inflammatory drugs, which can solve the problem of highly toxic reagents that do not conform to the concept of green chemistry , Not suitable for industrial production and other problems, to achieve the effect of high purity of synthetic products, easy purification and separation, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
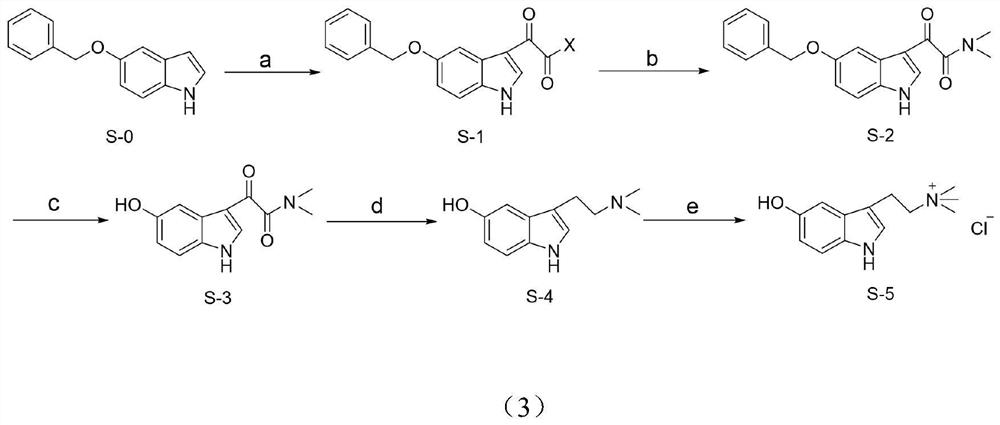
[0042] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-2-oxoacetyl chloride (S-1)
[0043] At room temperature, take a 100mL round-bottomed flask, add 2.7mL of anhydrous diethyl ether, oxalyl chloride (175μL, 2.0mmol) in sequence, and stir well, then add 5-benzyloxyindole (220mg, 1.0mmol) into the flask , after fully stirring for 30 minutes, the reaction solution was suction-filtered, and the filter cake was washed repeatedly with ether to obtain about 302.9 mg of a reddish-brown solid precipitate, which was the crude product of S-1, with a yield of 95%.
[0044]
[0045] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide (S-2)
[0046] At room temperature, take a 100mL round bottom flask, add potassium hydroxide (360mg, 6.38mmol) and 4.15mL water and dimethylamine hydrochloride (350mg, 4.25mmol) in turn, mix well, take S-1 (310mg , 1.0mmol) was slowly added to the mixed solution, stirred for 40 minutes, suction filtered, washed with anhydrous ether ...
Embodiment 2
[0062] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-2-oxoacetyl chloride (S-1)
[0063]At room temperature, take a 100mL round-bottomed flask, add 2.7mL of anhydrous diethyl ether, oxalyl chloride (175μL, 2.0mmol) in sequence, and stir well, then add 5-benzyloxyindole (220mg, 1.0mmol) into the flask , after fully stirring for 30 minutes, the reaction solution was suction-filtered, and the filter cake was washed repeatedly with ether to obtain about 299.8 mg of a reddish-brown solid precipitate, which was the crude product of S-1, with a yield of 94%.
[0064]
[0065] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide (S-2)
[0066] At room temperature, take a 100mL round-bottomed flask, add sodium hydroxide (170mg, 4.25mmol) and 4.15mL water and dimethylamine hydrochloride (350mg, 4.25mmol) in turn, mix well, take S-1 (310mg , 1.0mmol) was slowly added to the mixed solution, stirred for 40 minutes, suction filtered, washed with anhydrous ether to...
Embodiment 3
[0082] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-2-oxoacetyl chloride (S-1)
[0083] At room temperature, take a 100mL round-bottomed flask, add 2.7mL of anhydrous diethyl ether, oxalyl chloride (175μL, 2.0mmol) in sequence, and stir well, then add 5-benzyloxyindole (220mg, 1.0mmol) into the flask , after fully stirring for 40 minutes, the reaction solution was suction-filtered, and the filter cake was washed repeatedly with ether to obtain about 293.1 mg of a reddish-brown solid precipitate, which was the crude product of S-1, with a yield of 95%.
[0084]
[0085] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide (S-2)
[0086] At room temperature, take a 100mL round-bottomed flask, add sodium hydroxide (255mg, 6.38mmol) and 4.15mL water and dimethylamine hydrochloride (350mg, 4.25mmol) in turn, mix well, take S-1 (310mg , 1.0mmol) was slowly added to the mixed solution, stirred for 40 minutes, suction filtered, washed with anhydrous ether t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com