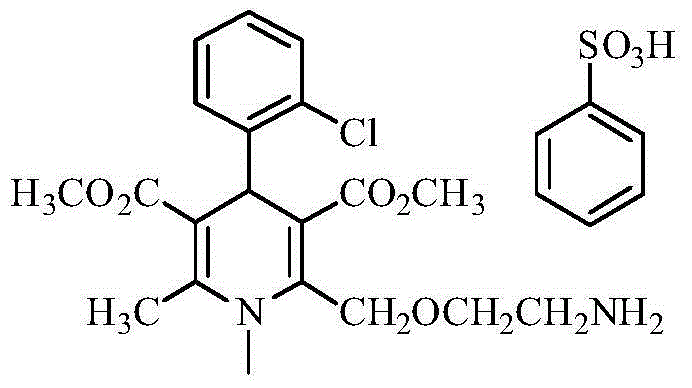
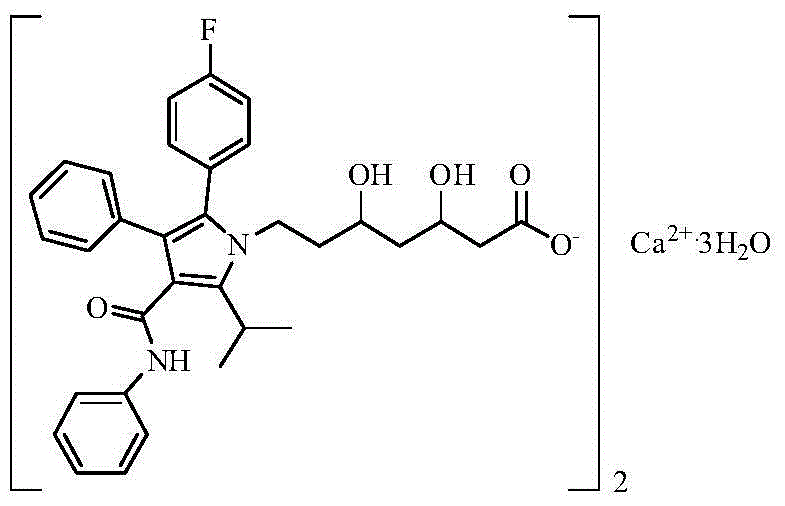
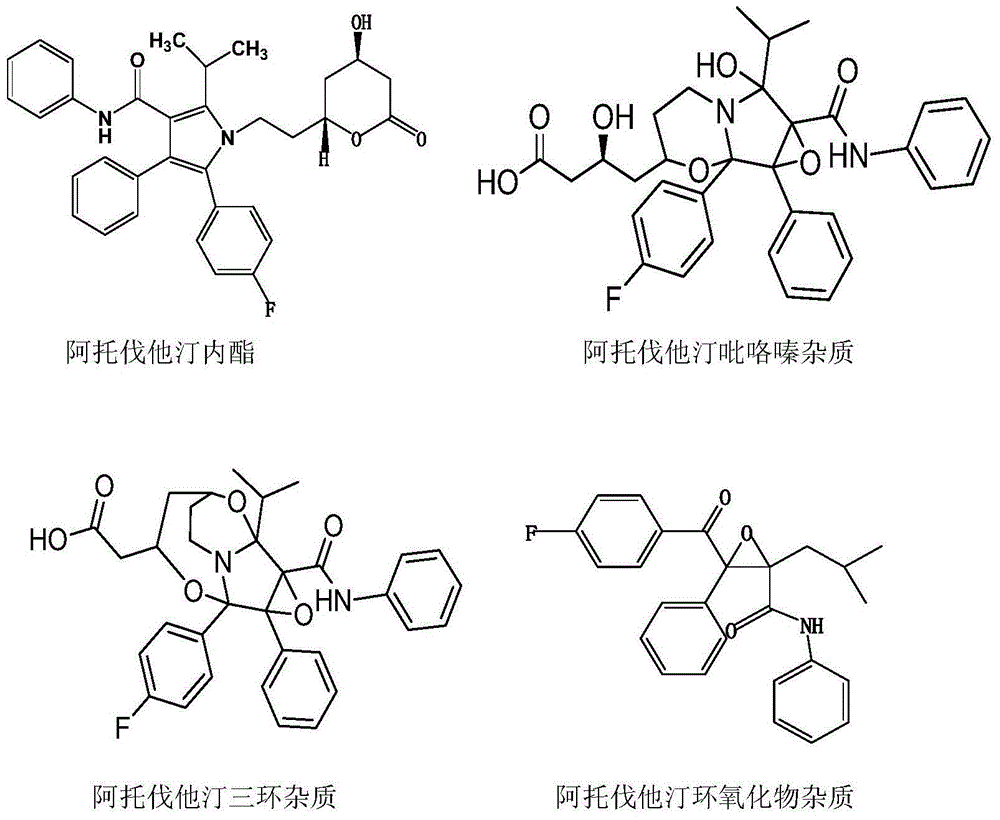
Preparation method of high-stability amlodipine atorvastatin calcium tablet
A technology of atorvastatin calcium and amlodipine, which can be used in pharmaceutical formulations, metabolic diseases, cardiovascular system diseases, etc., and can solve problems such as solubility and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Patents CN101185646A (prescription 1) and CN20152341U (prescription 2) describe the preparation of prescriptions, which are used together with the original research tablet for stability studies under accelerated conditions (40°C / 75%). Investigate the impact of conventional microcrystalline cellulose and different processes. The prescription list is as follows:
[0059]
[0060]
[0061] Preparation Process:
[0062] The preparation process of prescription 1 is the same as embodiment 2.
Embodiment 2
[0066] The present invention is also not limited to this embodiment for the prescription and technology of compound amlodipine atorvastatin calcium tablet. We selected microcrystalline cellulose with different peroxide contents from the market to prepare amlodipine atorvastatin calcium tablets; compared the results of three microcrystalline cellulose stability related substances, the results showed that the peroxide value of microcrystalline cellulose For the prescription of 35ppm, the oxidative degradation impurities are significantly reduced. The prescription list is as follows:
[0067]
[0068]
[0069] Preparation Process:
[0070] ① Raw and auxiliary materials batching process:
[0071] A. Accurately weigh croscarmellose sodium (internal added part), microcrystalline cellulose, pregelatinized starch, and hydroxypropyl cellulose according to the prescription quantity, put them into plastic bags separately, and mark them well.
[0072] B. The processing procedure ...
Embodiment 3
[0092] Embodiment 3, peroxide determination method in microcrystalline cellulose
[0093] A. Titanium trichloride-sulfuric acid test solution: Take 20ml of 15% titanium trichloride solution, carefully mix it with 13ml of sulfuric acid in an ice bath, add an appropriate amount of concentrated hydrogen peroxide solution until yellow appears, heat until white smoke is emitted, and put Cool, dilute with water repeatedly and evaporate until the solution is nearly colorless, add water to obtain a colorless solution, add water to 100ml, filter, and obtain.
[0094] B. Operate at 20-25°C, take 4.0g of microcrystalline cellulose, put it in a 100ml measuring bottle, add about 80ml of water, shake for 10 minutes, add water to the mark, shake well, filter, take 25ml of the filtrate, add three Titanium chloride-sulfuric acid test solution 2.0ml, shake well, stand for 30 minutes, as the test solution; take the above-mentioned continued filtrate 25ml, add 2.0ml of 13% (V / V) sulfuric acid sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com