Determining method of atorvastatin calcium related substance
A technology of atorvastatin calcium and a determination method is applied in the field of determination of pharmaceutical impurities, and can solve the problems of difficult detection and separation of impurity E, difficulty in separation of impurity B, and inability to control product quality well.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Assay method, the steps are as follows:
[0094] Step 1, preparation of the test solution:
[0095] Take an appropriate amount of atorvastatin calcium, dissolve it in a solvent [acetonitrile-tetrahydrofuran-water (1:1:2)] and quantitatively dilute to make a solution containing about 1mg in every 1ml, as the test solution;
[0096] Step 2, the preparation of mixed reference solution:
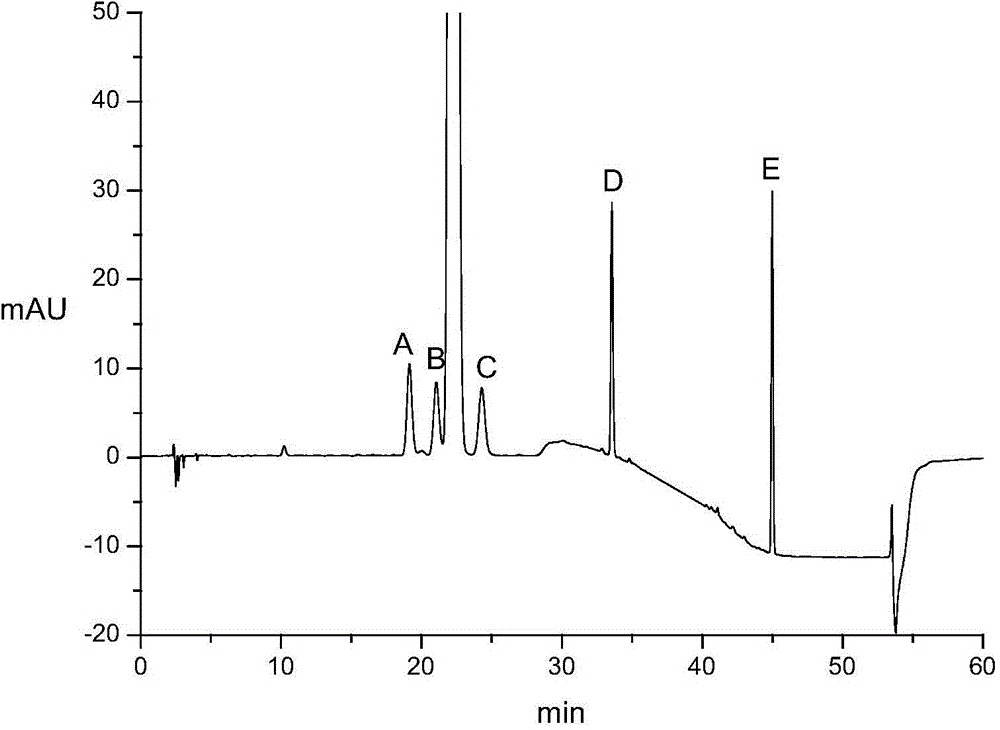
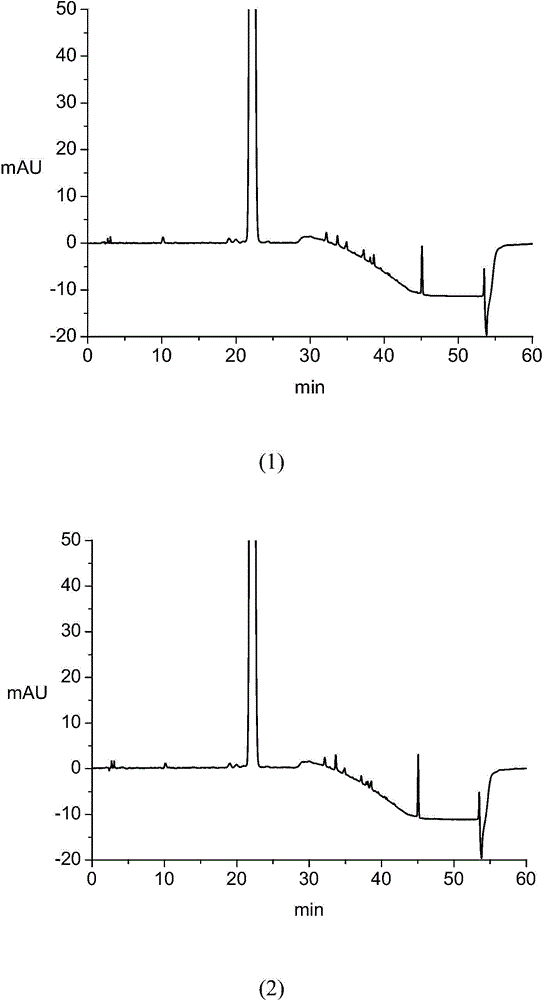
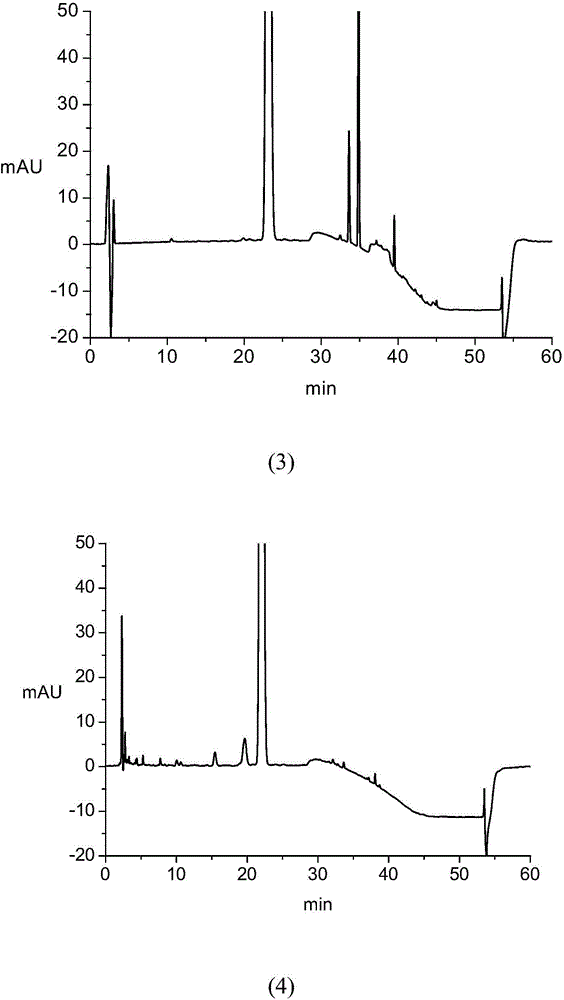
[0097] Accurately weigh the appropriate amount of impurity A reference substance, impurity B reference substance, impurity C reference substance, impurity D reference substance, impurity E reference substance and atorvastatin calcium reference substance, add the above solvent to dissolve and quantitatively dilute to make 1ml A solution containing approximately 3 μg of impurity A, 2 μg each of impurity B, impurity C, impurity D, and impurity E, and 10 μg of atorvastatin calcium is used as a mixed reference solution. Step 3, high performance liquid chromatography HPLC analysis:
[0098] T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com