Determination method of related substances in atorvastatin calcium capsules
A technology for the detection of atorvastatin calcium, which is applied in the field of separation of drugs into components for testing or analysis by column chromatography, which can solve problems such as threats to clinical drug safety and achieve the effect of improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
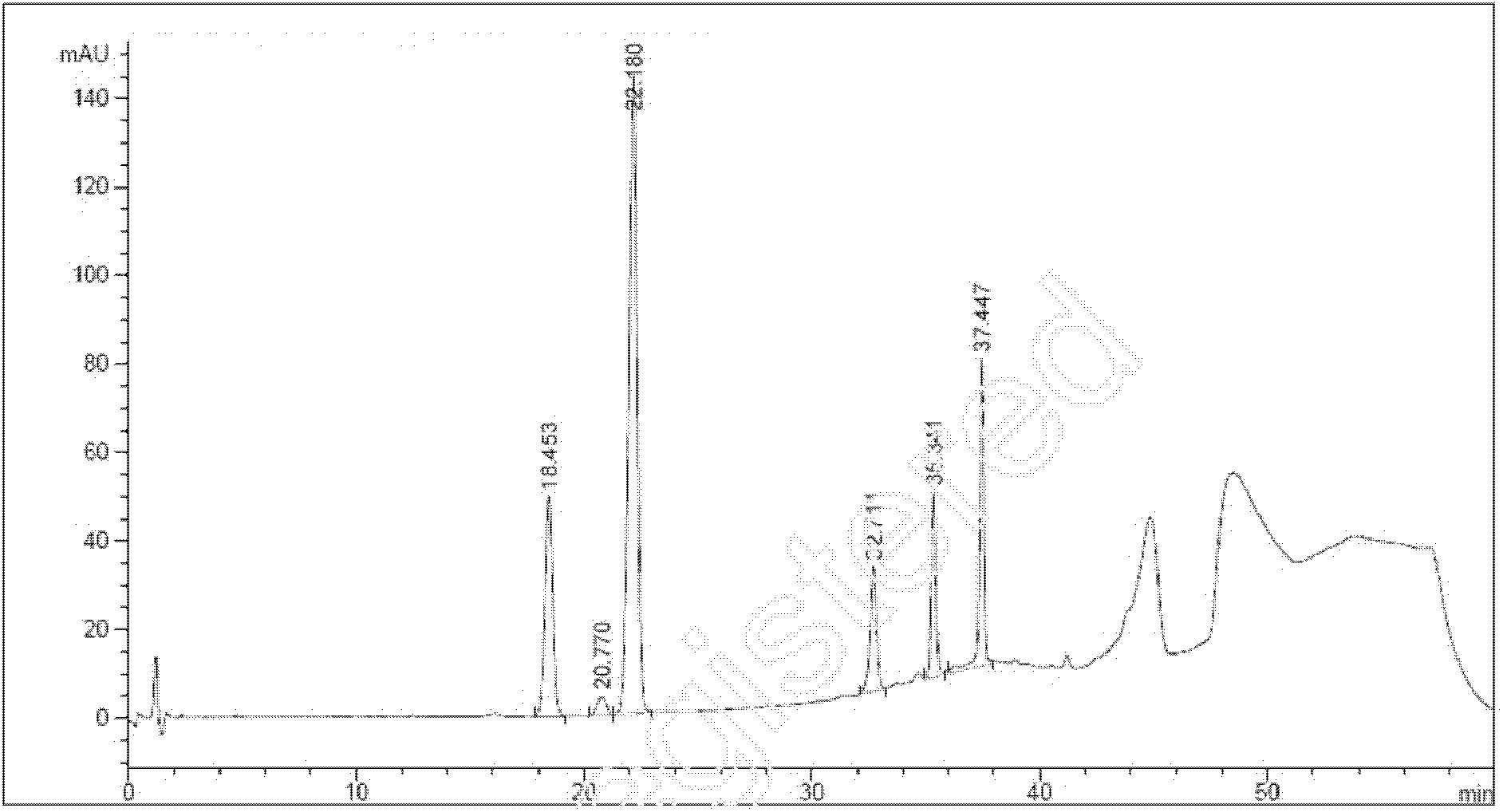
[0073] Embodiment 1 The establishment of detection method
[0074] 1 Chromatographic conditions
[0075] A Kromasil C-18 chromatographic column was selected, with a particle size of 5 μm, a column length of 250 mm, and a column diameter of 4.6 mm, using an Agilent 1200 chromatographic workstation.
[0076] With acetonitrile-tetrahydrofuran (92.5:7.5, v / v) as mobile phase A, 0.56% ammonium dihydrogen phosphate solution-mobile phase A (58:42, v / v) as mobile phase B, 0.56% ammonium dihydrogen phosphate solution -Mobile phase A-methanol (100:100:300, v / v) is used as mobile phase C, the detection wavelength is 246nm, the column temperature is 25°C, and the elution conditions are:
[0077] From 0 min to 20 min, the mobile phase is B, and the flow rate is 1.8ml / min; from the 20 min to the 35 min, the mobile phase gradient changes to B and C with a volume ratio of 25%:75%, B+C=100%, and the flow rate 1.5ml / min; from the 35th minute to the 40th minute, keep the proportion of the mobi...
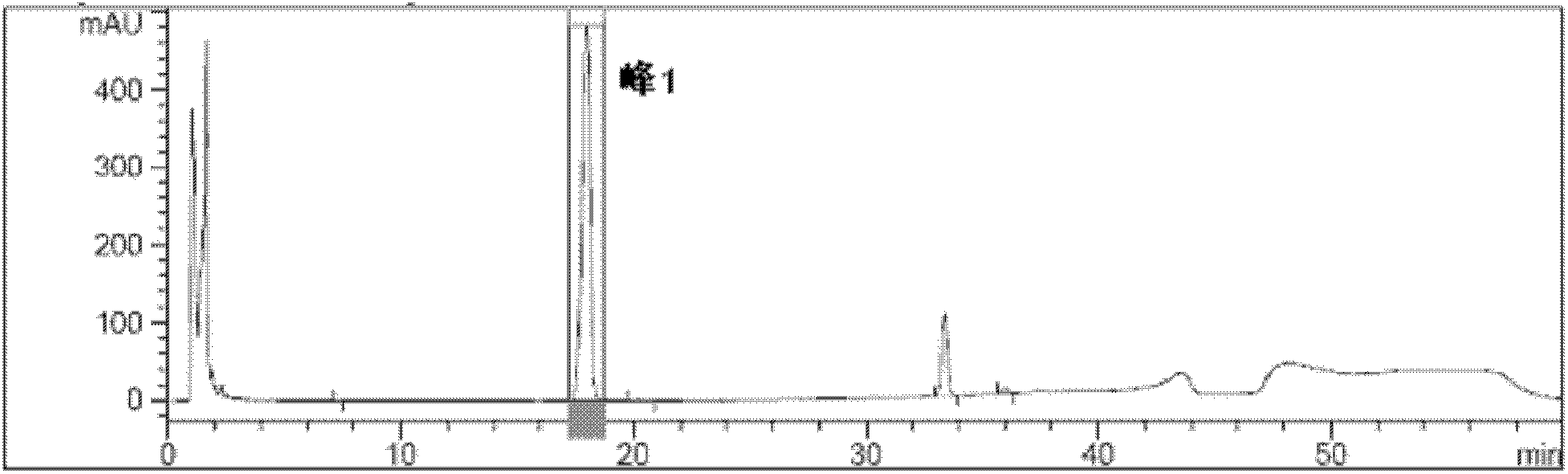
Embodiment 2
[0105]Adopt Kromasil C-18 chromatographic column, particle diameter 5 μm, column length 250mm, column diameter 4.6mm; Adopt Agilent 1100 chromatographic workstation; With acetonitrile-tetrahydrofuran (92:8, v / v) as mobile phase A, 0.56% dihydrogen phosphate Ammonium solution-mobile phase A (56:44, v / v) was used as mobile phase B, 0.56% ammonium dihydrogen phosphate solution-mobile phase A-methanol (100:100:300, v / v) was used as mobile phase C, according to The following table carries out gradient elution, the detection wavelength is 240nm, and the column temperature is 20°C, wherein 0.56% in 0.56% ammonium dihydrogen phosphate solution refers to the mass volume ratio, and the elution conditions are as follows:
[0106] From 0 min to 17 min, the mobile phase is B, and the flow rate is 1.8ml / min; from 17 min to 32 min, the mobile phase gradient changes to B and C with a volume ratio of 25%:75%, B+C=100%, and the flow rate 1.5ml / min; from the 32nd to the 40th minute, keep the rat...
Embodiment 3
[0112] Adopt Discovery C-18 chromatographic column, particle diameter 5 μm, column length 250mm, column diameter 4.6mm; Adopt Shimadzu LC-2010AHT chromatographic workstation; With acetonitrile-tetrahydrofuran (95:5, v / v) as mobile phase A, 0.56% Ammonium dihydrogen phosphate solution-mobile phase A (60:40, v / v) as mobile phase B, 0.56% ammonium dihydrogen phosphate solution-mobile phase A-methanol (100:100:300, v / v) as mobile phase C, carry out gradient elution, detection wavelength is 250nm, column temperature is 30 ℃, wherein 0.56% in 0.56% ammonium dihydrogen phosphate solution refers to mass volume ratio, and elution condition is as follows:
[0113] From 0 min to 20 min, the mobile phase is B, and the flow rate is 1.8ml / min; from the 20 min to the 35 min, the mobile phase gradient changes to B and C with a volume ratio of 22%:78%, B+C=100%, and the flow rate From the 35th minute to the 41st minute, keep the ratio of the mobile phase unchanged, and the flow rate is 1.5ml / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com