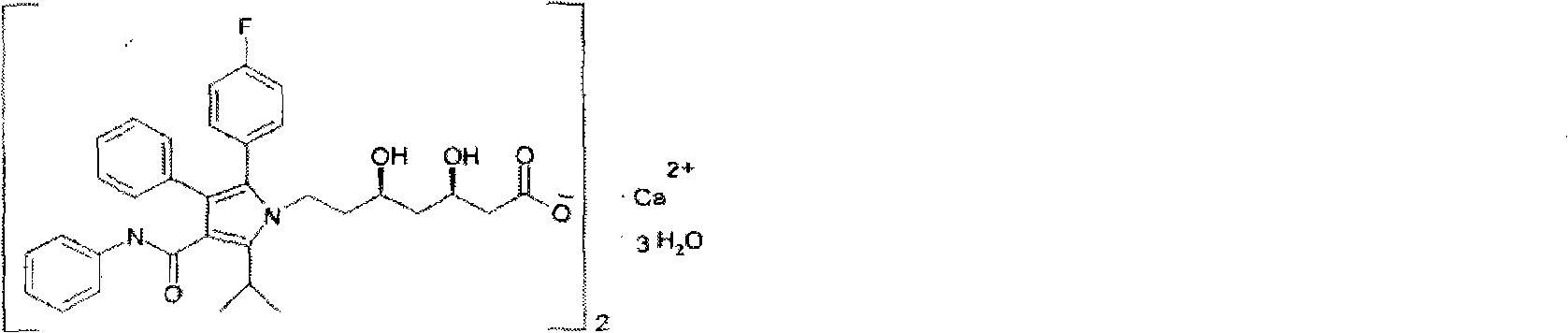Atorvastatin calcium oral disintegrating tablet and preparation method thereof
A technology of atorvastatin calcium and orally disintegrating tablets, which is applied in the field of orally disintegrating tablets, can solve the problems of high encapsulation rate, poor production feasibility, large particle size of pellets or particles, and achieve short disintegration time, The effect of convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 40g of atorvastatin calcium, 60g of gastric-soluble polyacrylic acid resin EPO and 2g of diethyl phthalate and dissolve them in 20ml of absolute ethanol, stir to dissolve and suspend evenly, pour into the mixture at a stirring speed of 400-600rpm , the concentration is 0.01M NaOH aqueous solution, after the precipitation is complete, suction filtration, the obtained powder is dried in a vacuum oven for 24 hours, taken out, and stored in a closed container in a cool place to obtain atorvastatin calcium taste-masked microcapsules.
[0023] Tablet prescription:
[0024] Atorvastatin Calcium Taste Masked Microcapsules 25g
[0025] Mannitol 100g
[0026] L-HPC 15g
[0028] Micronized silica gel 1g
[0029] Magnesium stearate 0.5g
[0030] Weigh the above materials (except magnesium stearate) into a three-dimensional mixer and mix for 30 minutes, then add magnesium stearate and mix for 1 to 2 minutes; pour the mixed materials into th...
Embodiment 2
[0032]Weigh 20g of atorvastatin calcium, 80g of gastric-soluble polyacrylic acid resin EPO and 2g of diethyl phthalate and dissolve in 20ml of absolute ethanol, stir to dissolve and suspend evenly, pour in and stir at a speed of 400-600rpm After the precipitation is complete, filter with suction, place the obtained powder in a vacuum drying oven to dry for 24 hours, take it out, and store it in an airtight container in a cool place to obtain the taste-masked microcapsules of atorvastatin calcium.
[0033] Tablet prescription:
[0034] Atorvastatin Calcium Taste Masked Microcapsules 50g
[0035] Mannitol 160g
[0036] L-HPC 10g
[0037] Croscarmellose Sodium 10g
[0038] Calcium carbonate 10g
[0039] Micronized silica gel 2g
[0040] Flavoring agent 0.01g
[0041] Magnesium stearate 0.5g
[0042] Weigh the above materials (except magnesium stearate) into a three-dimensional mixer and mix for 30 minutes, then add magnesium stearate and mix for 1 to 2 minutes; pour the mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com