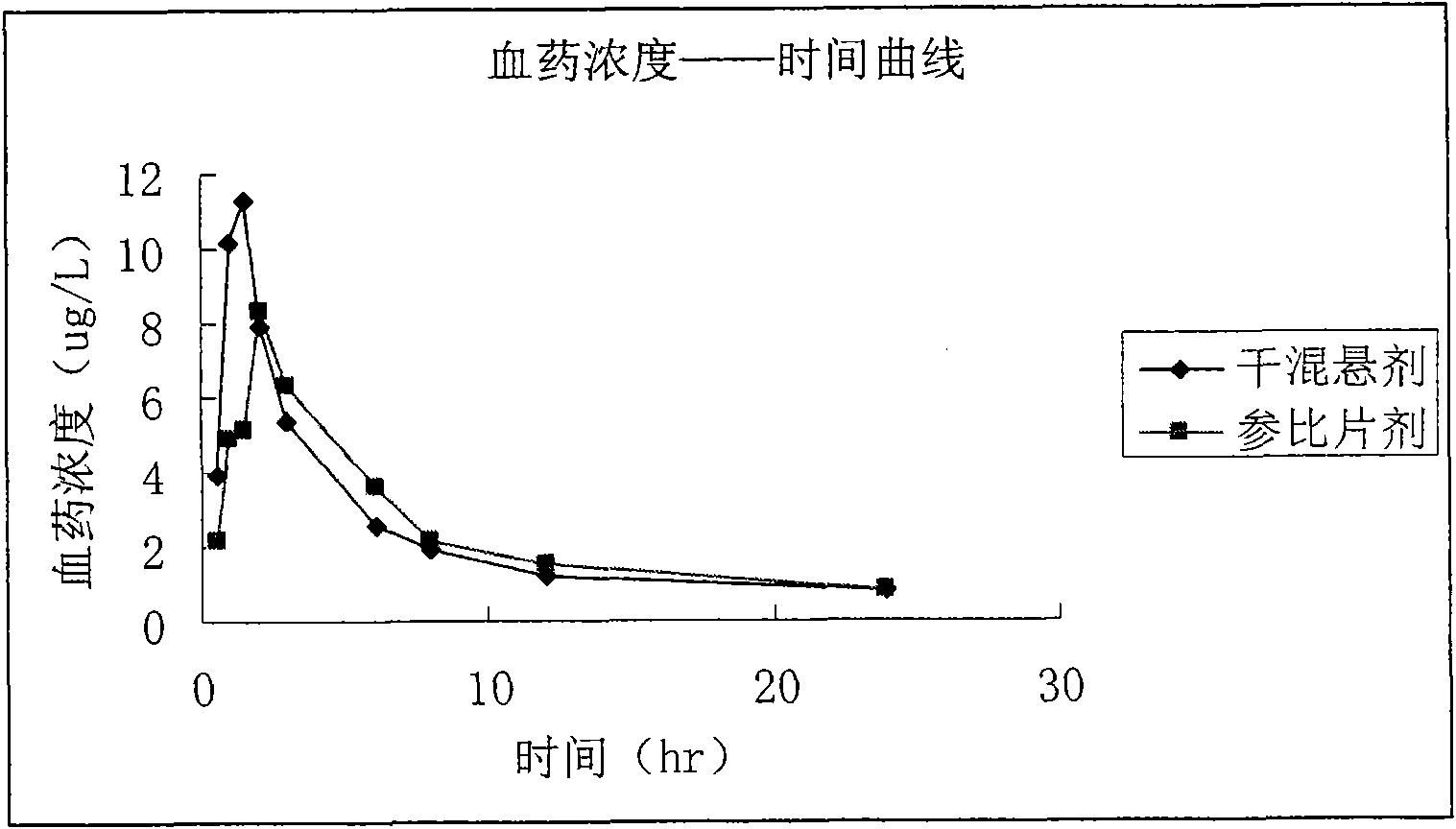
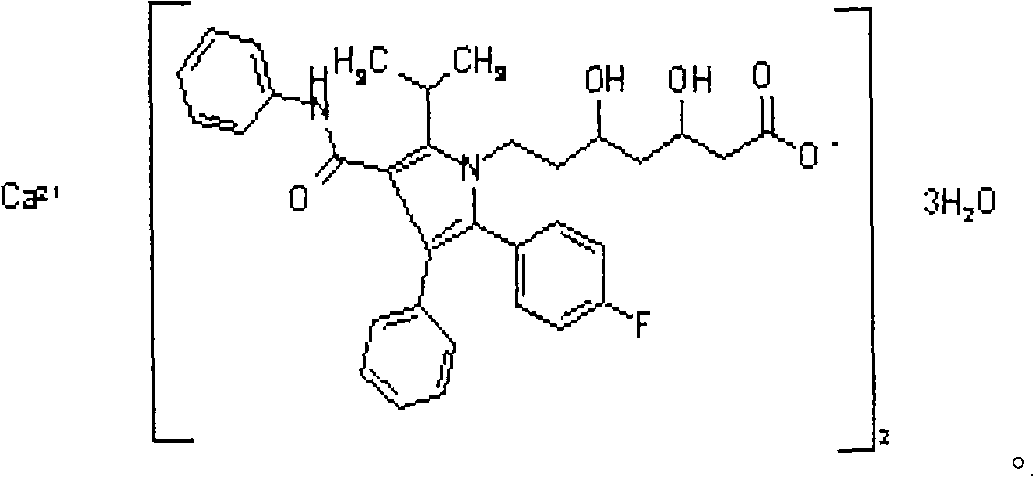
Atorvastatin calcium dried suspension and preparation method thereof
A technology of atorvastatin calcium and dry suspension, which is applied in pharmaceutical formulations, drug delivery, metabolic diseases, etc., can solve the problems of clinical drug effect, difficulty in swallowing, low solubility, etc., and achieves good compliance and avoidance difficulties. Swallow, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the present invention preferably also includes: passing atorvastatin calcium through a 120 mesh sieve before step (1), and passing filler, suspending agent and / or suspending agent, flavoring agent, and lubricant 100 mesh sieve steps.
[0027] In the preparation method of the present invention, the mixing in step (1) is preferably carried out by crossing a 60 mesh-80 mesh sieve; the granulation in step (3) is preferably carried out by crossing a 18-24 mesh sieve; step ( The drying in 4) is preferably carried out by blast drying at 50°C until the water content in the granules is less than or equal to 1 part by weight; the granulation in step (5) is preferably carried out by passing the dried granules 18-24 mesh sieve method.
[0028] In the preparation method of the present invention, the wetting agent is preferably 40%-60% by volume ethanol aqueous solution.
[0029] In the preparation method of the present invention, before atorvastatin calci...
Embodiment 1
[0044] Pass atorvastatin calcium through a 120-mesh sieve, and pass the rest of the auxiliary materials through a 100-mesh sieve.
[0045] Take by weighing 10.0g (calculated as atorvastatin) atorvastatin calcium after sieving as main ingredient; take by weighing 10g sodium carboxymethylcellulose as suspending agent and / or suspending agent; 200g lactose as filler ; 2g aspartame, 30g stevioside and 3g citric acid as flavoring agent. Mix these materials evenly through a 60 mesh-80 mesh sieve, add 100ml of 50% by volume ethanol aqueous solution to the resulting mixture as a wetting agent to make a soft material, then granulate through a 18-24 mesh sieve, and then drum under 50°C. Air drying until the moisture content in the obtained material is less than or equal to 1 part by weight.
[0046] The dried material is passed through a 18-24 mesh sieve for granulation, and 2 g of magnesium stearate is added as a lubricant, mixed evenly, and packaged to obtain the atorvastatin calcium ...
Embodiment 2
[0048] Prepare the atorvastatin calcium dry suspension of embodiment 2 according to the method identical with embodiment 1, difference is to take by weighing 20.0g (calculated as atorvastatin) atorvastatin calcium as principal agent; Sodium methylcellulose and 10g crospovidone are used as suspending agent and / or suspending agent; 400g lactose is used as filler; 3g aspartame, 40g stevioside and 4g citric acid are used as flavoring agents; and 150l 50% by volume of ethanol aqueous solution is used as a wetting agent, and 4 g of magnesium stearate is used as a lubricant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com