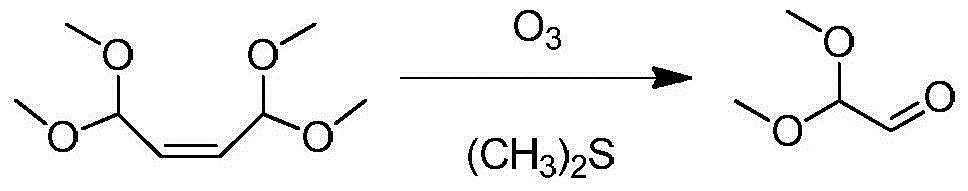Synthesis method for chiral intermediate of atorvastatin calcium
A technology of atorvastatin calcium and chiral intermediates, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of difficult preservation of enzymes, difficulty in industrial production, and easy inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
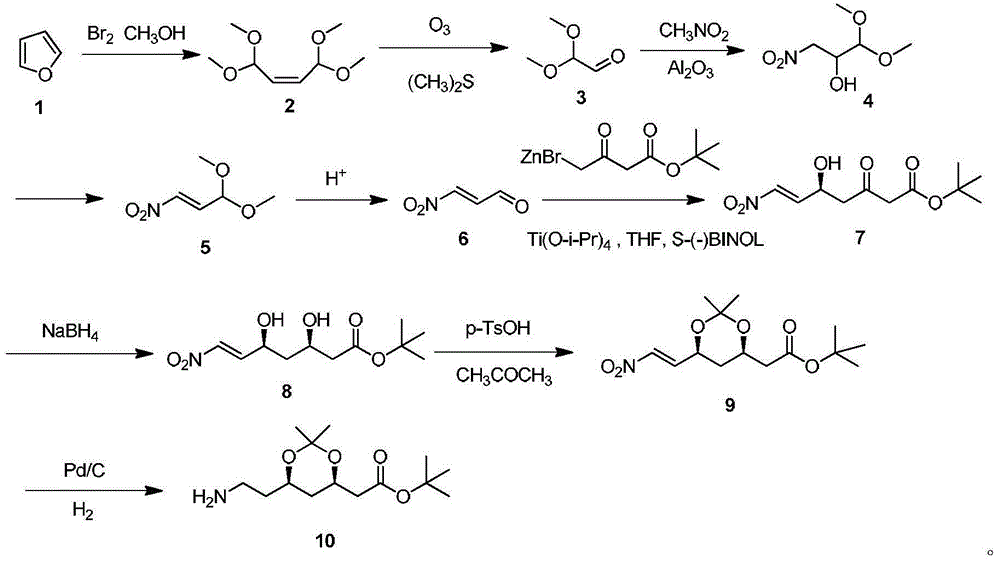
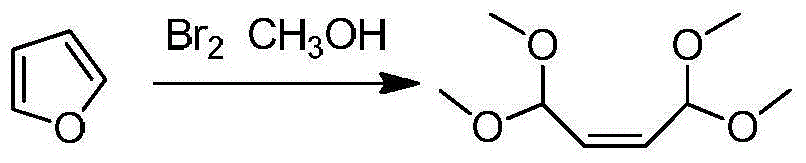
[0033]In a 2000mL reaction flask equipped with mechanical stirring and a thermometer, add 50g (0.735mol) of furan and 1000mL of methanol, place it at -50°C, replace the air with nitrogen protection, and slowly add 240g (1.5mol) of liquid bromine dropwise. After the dropwise addition, react for 3 hours, raise the temperature to 0°C and continue the reaction for 2 hours. After the reaction is completed, rise to room temperature, adjust the pH of the reaction solution to 7-8 with 1000mL saturated sodium bicarbonate solution, distill off the remaining methanol under reduced pressure, and then use ethyl acetate Extract the reaction solution three times with 1000 mL of ester, combine the organic phases and wash with saturated sodium chloride solution, separate the organic phase, and distill off the ethyl acetate to obtain 75 g of compound 1,1,4,4-tetramethoxy-2-butene .
Embodiment 2
[0035]
[0036] Add 70g of compound 1,1,4,4-tetramethoxy-2-butene and 500mL of dimethyl sulfide into a 1000mL reaction bottle with mechanical stirring and a thermometer, place it at -10°C, and replace it in the bottle Nitrogen was introduced after the air, and 30g of ozone was slowly injected into the closed reaction system, and the reaction temperature was controlled to maintain at -10°C. After 10 hours of reaction, the raw materials were completely reacted, raised to room temperature, and the compound 1,1-dimethoxy was obtained after distilling off the solvent. Acetaldehyde 32g.
Embodiment 3
[0038]
[0039] In a 1000mL reaction flask with mechanical stirring and a thermometer, add 30g (0.3mol) of 1,1-dimethoxyacetaldehyde, 19g (0.3mol) of nitromethane and 3g (0.03mol) of anhydrous aluminum oxide into 500mL of DMF , under nitrogen protection, heated to 130 ° C, after the reaction was completed, lowered to room temperature, filtered the reaction liquid, added 500 mL of saturated sodium chloride solution to the filtrate after concentration, then extracted the reaction liquid twice with 600 mL of dichloromethane, combined organic 43 g of 1,1-dimethoxy-2-hydroxy-3-nitropropane was obtained after distilling off the solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com