Method for preparing amorphous atorvastatin calcium
An atorvastatin calcium and amorphous technology is applied in the field of chemistry, which can solve the problems of long time for releasing tert-butyl group, difficult separation of the final product, low product purity, etc., and achieves shortened preparation period, good product quality and synthetic yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
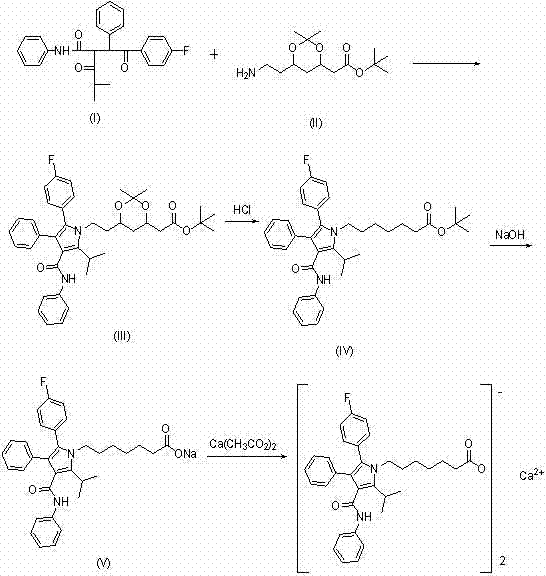
[0017] Example 1: Compound III (4R-CIS)-6-[2-2[-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-(anilinecarbonyl) - Preparation of tert-butyl 1H-pyrrol-1-ylbutylethyl]-2,2-dimethyl-1,3-dioxolane-4-acetate.
[0018] Put 72.0g of compound I and 50g of compound II into a flask, add 250ml of n-heptane, 90ml of tetrahydrofuran, and 4.2g of pivalic acid, heat up and reflux at 80-100°C to separate water for about 28h, after the reaction is complete, recover the solvent to dryness under reduced pressure, add 200ml of methanol was heated to reflux to dissolve, cooled to 0-5°C, crystallized for 1.0h, centrifuged and dried to obtain 78.0g of compound III. HPLC≥99%, molar yield 0.92.
Embodiment 2
[0019] Example 2: Preparation of amorphous atorvastatin calcium.
[0020] Add 20.0g of compound III to the reaction bottle, use 100ml of methanol and 20ml of THF as solvents, heat up to 30°C to dissolve, add 17.0g of 1.5N hydrochloric acid, keep it at 25-30°C for 2.0-3.0h, HPLC traces the completion of the reaction of the material, and cools down to At about 10°C, add 15.0g of 15% NaOH, keep it at 20-25°C for 2.0-3.0h, and track it by HPLC until the reaction is complete. Add 80ml of n-hexane and 120ml of water, stir for 10min, separate the phases, extract the water phase with 80ml of n-hexane again, add 9.0g of calcium acetate and 20ml of purified water to the water phase, stir for 1.0h, then add 100ml of ethyl acetate Extract, concentrate the organic phase to dryness under reduced pressure, add 100ml of acetone and stir to dissolve, distill under reduced pressure at an internal temperature ≦50°C, concentrate to 40ml, pour into a watch glass, and dry under reduced pressure at ...
Embodiment 3
[0021] Example 3: Preparation of amorphous atorvastatin calcium.
[0022] The ethyl acetate in the step of embodiment 2 is replaced with propyl acetate, and others are the same as embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com