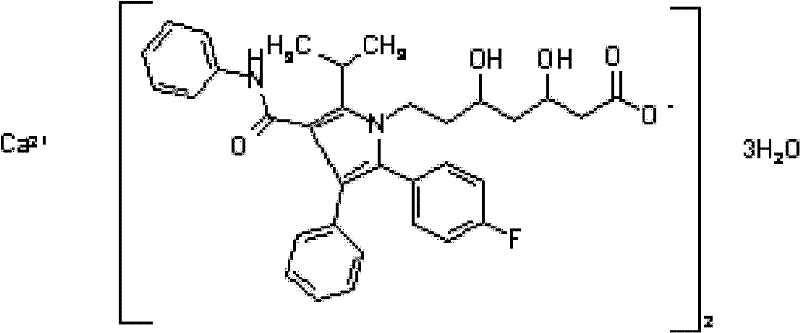
Atorvastatin calcium tablet
A technology of atorvastatin calcium and tablets, which is applied in the directions of pill delivery, metabolic diseases, active ingredients of heterocyclic compounds, etc., can solve the problems of increased drug production cost, poor tablet stability, indigestion, etc. The effect of rapid dissolution, stable quality and low packaging cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0052] Example 5 Stability Study of Atorvastatin Calcium Tablets
[0053] The tablets (specification 20mg) prepared in Examples 1-4 of the present invention and the tablets (control group) prepared in Example 1 of CN1911209A were placed under accelerated conditions (temperature 40°C, relative humidity 75% ± 5 %), the stability study was carried out for 6 months.
[0054] The detection method of related substances is in accordance with high performance liquid chromatography (Appendix VD of Chinese Pharmacopoeia 2010 Edition). Among them, chromatographic conditions and system adaptability test: use octylsilane bonded silica gel as filler, acetonitrile-0.05mol / L citric acid (50:50) (adjust pH 4.0 with ammonia water) as mobile phase, detection wavelength 244nm, the number of theoretical plates is not less than 4000 based on atorvastatin. Determination method: Take an appropriate amount of fine powder of this product (approximately equivalent to C 33 h 35 FN 2 o 5 12.5mg), pu...
Embodiment 6
[0058] Example 6 Disintegration time limit and dissolution rate investigation of atorvastatin calcium tablet
[0059] (1) Determination of disintegration time limit
[0060] Take 6 atorvastatin calcium tablets (specification 20mg) prepared in Examples 1-4 of the present invention, put them in a 250ml beaker, add 100ml of water at 15-25°C, shake for 3 minutes, and measure the disintegration time limit. See Table 2 for test results.
[0061] sample Sheet hardness (N) Disintegration time limit (s) Example 1 55-60 68-74 Example 2 53-58 71-75 Example 3 55-59 72-81 Example 4 54-56 68-76
[0062] According to the test results in Table 2, it can be seen that the hardness of the atorvastatin calcium tablet prepared by the present invention reaches 50-60N, and in 15-25°C water, it disintegrates completely within 80s and passes through the No. 2 sieve. This shows that the tablet of the present invention disinte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com