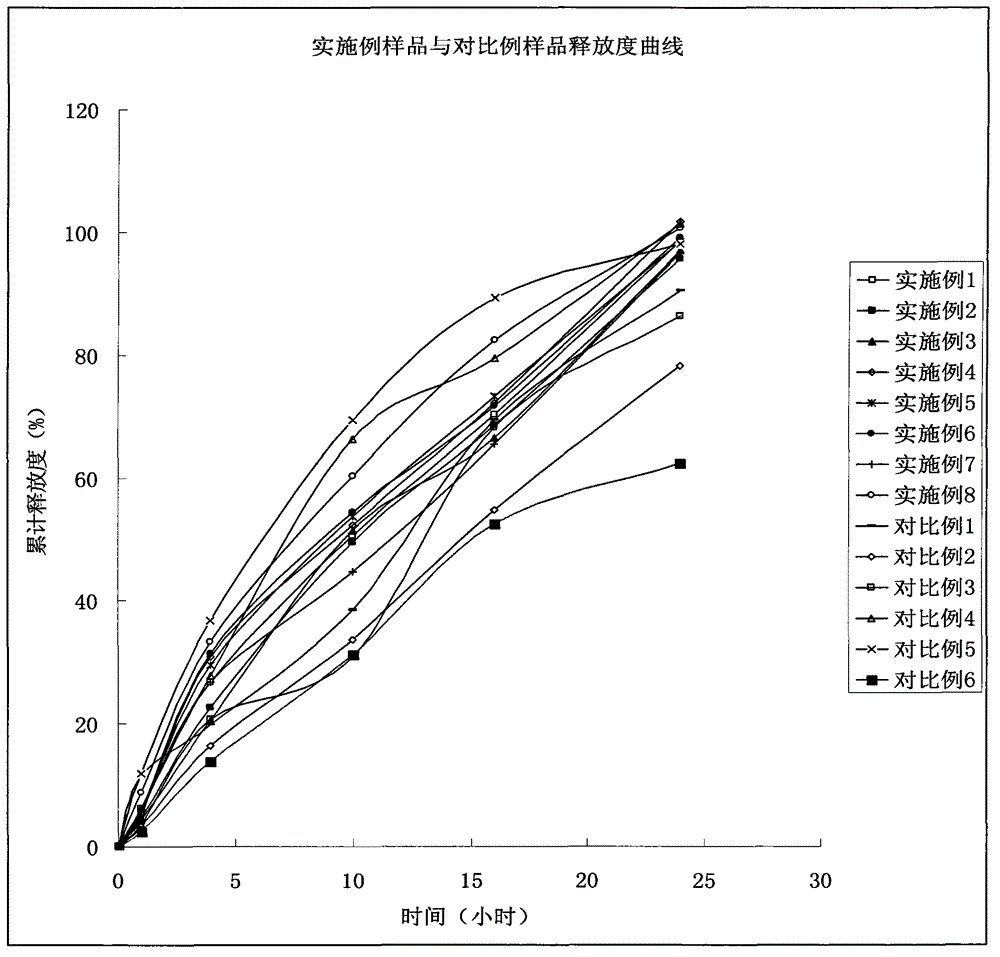
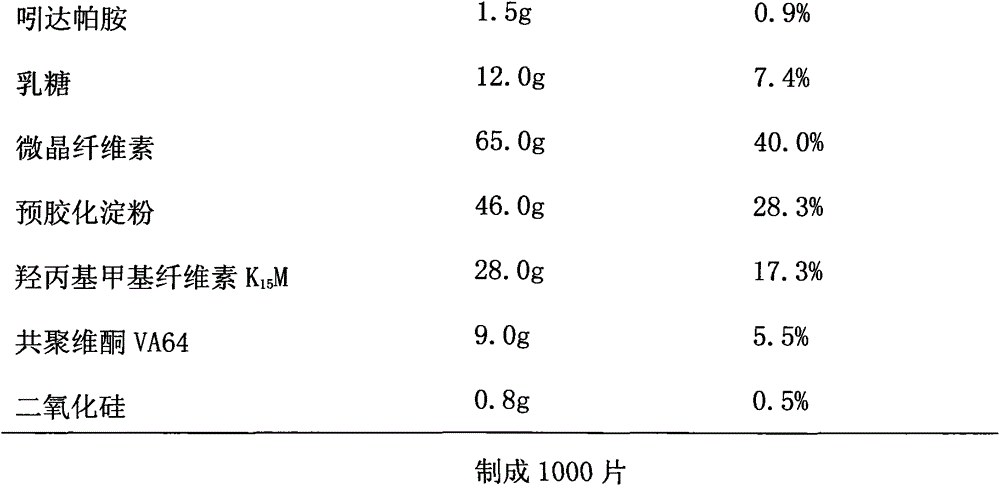
Indapamide sustained-release drug composite and preparation method thereof
A technology for indapamide and sustained-release drugs, applied in the field of medicine, can solve the problems of unable to guarantee the normal release of drugs, difficult to achieve the effect of sustained-release, difficult to play a sustained-release effect, etc., and achieves good release uniformity and improved Drug bioavailability, the effect of avoiding high blood concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] prescription
[0027]
[0028] Preparation:
[0029] (1) The active ingredient indapamide of the prescribed amount is ultrafinely pulverized, and the particle size is controlled to be less than 50 μm.
[0030] (2) Take the prescribed amount of lactose, microcrystalline cellulose, pregelatinized starch, hydroxypropyl methylcellulose, copovidone VA64, and magnesium stearate to pass through an 80-mesh stainless steel screen respectively.
[0031] (3) Take the pulverized indapamide and the lactose of the prescription amount and mix them uniformly, and then mix them with pregelatinized starch and microcrystalline cellulose to form a mixture A;
[0032] (4) Take by weighing prescription quantity VA64, hydroxypropyl methylcellulose K4M, magnesium stearate and mix with mixture A, form mixture B;
[0033] (5) Take the above-mentioned mixture B, and use No. Φ7 shallow punching tablet to obtain it.
Embodiment 2
[0035] prescription
[0036]
[0037] Preparation:
[0038] (1) The active ingredient indapamide of the prescribed amount is ultrafinely pulverized, and the particle size is controlled to be less than 50 μm.
[0039] (2) Take the prescribed amount of lactose, microcrystalline cellulose, pregelatinized starch, hydroxypropyl methylcellulose K15M, copovidone VA64, and magnesium stearate to pass through an 80-mesh stainless steel sieve respectively.
[0040] (3) Take the pulverized indapamide and the lactose of the prescription amount and mix them uniformly, and then mix them with pregelatinized starch and microcrystalline cellulose to form a mixture A;
[0041] (4) Take by weighing recipe quantity VA64, hydroxypropyl methylcellulose K15M, magnesium stearate and mix with mixture A, form mixture B;
[0042] (5) Take the above-mentioned mixture B, and use No. Φ7 shallow punching tablet to obtain it.
Embodiment 3
[0044] prescription
[0045]
[0046]
[0047] Preparation:
[0048] (1) The active ingredient indapamide of the prescribed amount is ultrafinely pulverized, and the particle size is controlled to be less than 50 μm.
[0049] (2) Take the prescribed amount of microcrystalline cellulose, pregelatinized starch, hydroxypropyl methylcellulose K4M, copovidone VA64, and magnesium stearate to pass through an 80-mesh stainless steel screen respectively.
[0050] (3) Take the pulverized indapamide and the precrossed starch of the prescription amount and mix them uniformly, and then mix them with microcrystalline cellulose to form a mixture A;
[0051] (4) Take by weighing prescription quantity VA64, hydroxypropyl methylcellulose K4M, magnesium stearate and mix with mixture A, form mixture B;
[0052] (5) Take the above-mentioned mixture B, and use No. Φ7 shallow punching tablet to obtain it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com