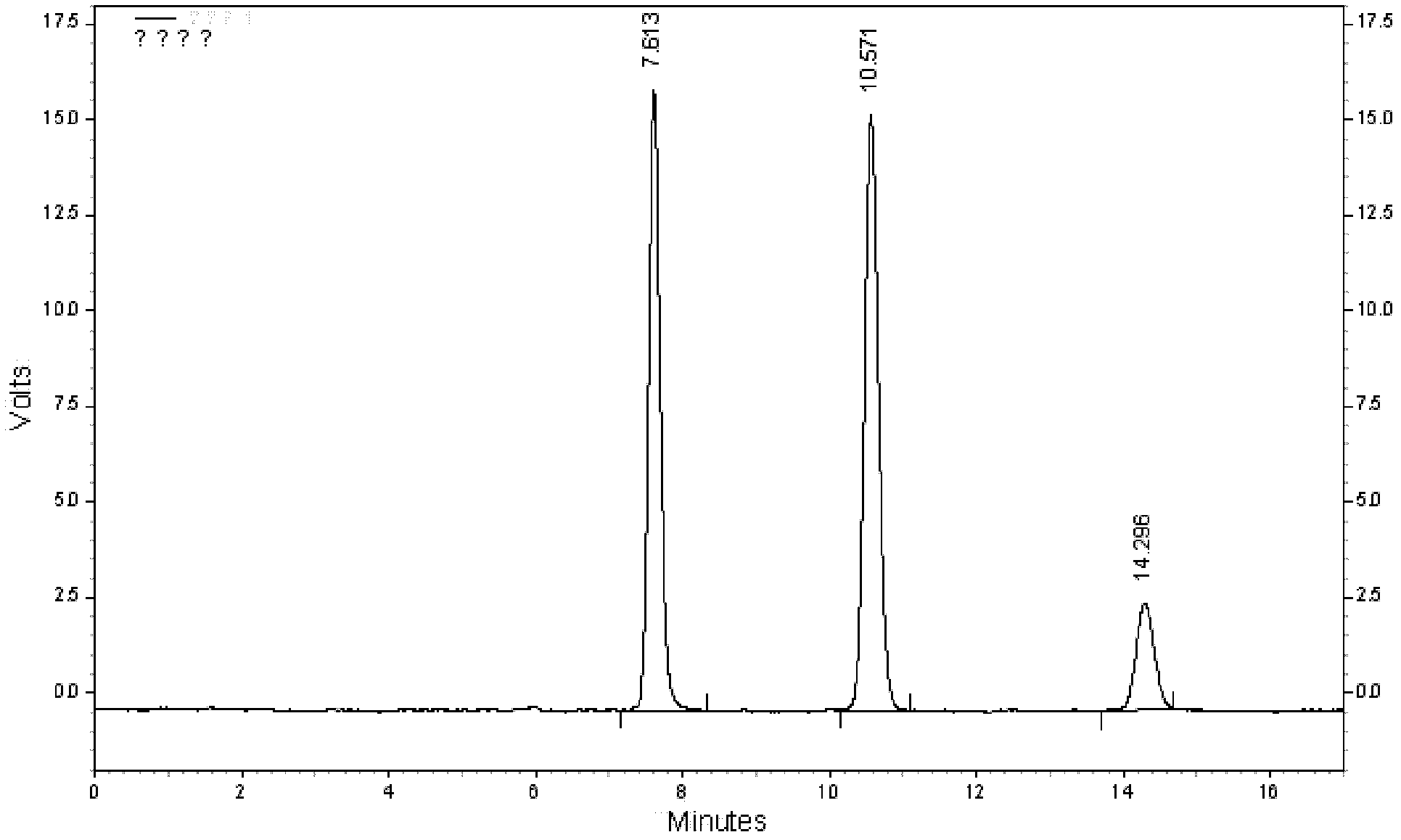
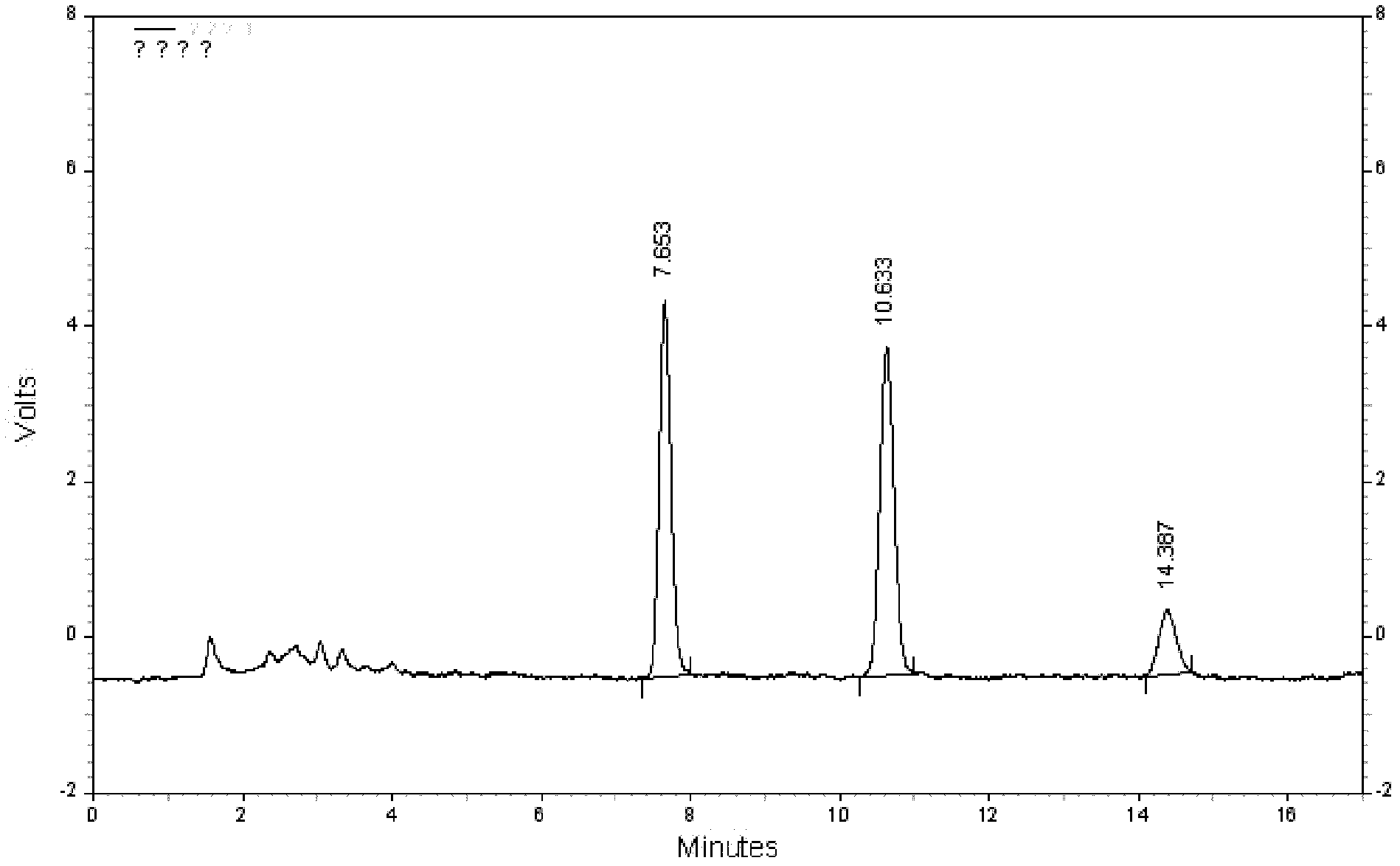
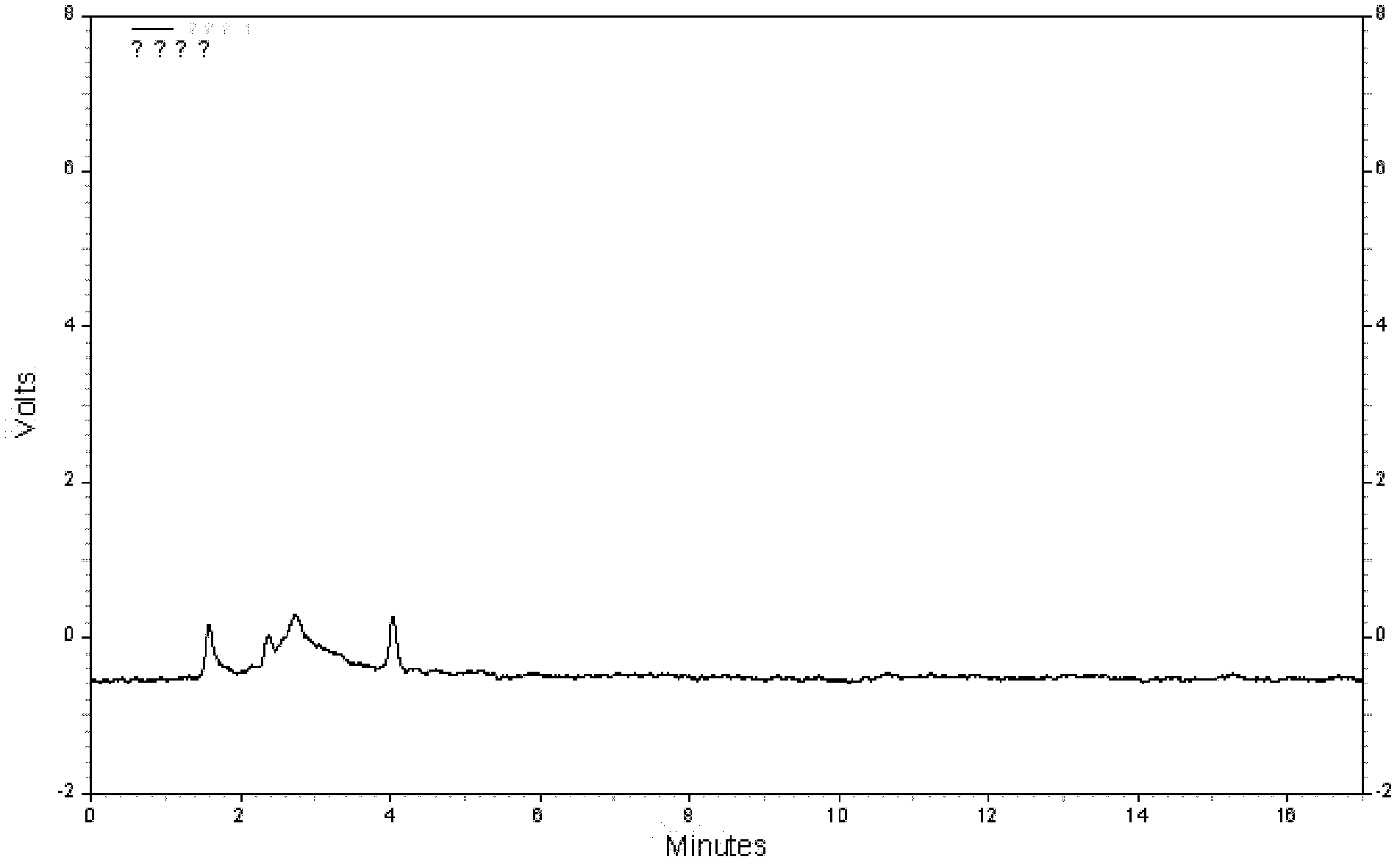
Qingpeng ointment and quality control method of Qingpeng ointment preparation
A Qingpeng, ointment technology, applied in the field of medicine, can solve the problems of inaccurate quality control of Qingpeng ointment products, weak quality control methods, quality standards to be improved, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Embodiment 1: the quality control method of Qingpeng ointment
[0117] Identification:
[0118] a. Identification of sub-rhubarb
[0119] Take 5g of Qingpeng ointment, add 5g of diatomaceous earth, add 50ml of methanol, sonicate for 30min, filter, take 25ml of filtrate, evaporate to dryness, add 30ml of water to dissolve the residue, add 3ml of hydrochloric acid, heat and reflux for 30min, cool, and extract twice with ether , 30ml each time, combined ether solution, evaporated to dryness, and dissolved the residue in 2ml of chloroform, as the test solution. Take another 0.5g sub-rhubarb reference medicinal material, add methanol 50ml, sonicate for 30min, filter, take 25ml of the filtrate, evaporate to dryness, add 30ml of water to dissolve the residue, add 3ml of hydrochloric acid, heat and reflux for 30min, cool, and extract with ether twice, each time 30ml, combined ether solution, evaporated to dryness, the residue was dissolved in 5ml of chloroform, and used as th...
Embodiment 2
[0131] Embodiment 2: the quality control method of Qingpeng ointment
[0132] Identification:
[0133] a. Identification of sub-rhubarb
[0134] Take 3-10g of Qingpeng ointment, add 5g of diatomaceous earth, add 55ml of methanol, sonicate for 25min, filter, take 25ml of filtrate, evaporate to dryness, add 30ml of water to dissolve the residue, add 3ml of hydrochloric acid, heat and reflux for 35min, cool, and extract with ether 2 times, 30ml each time, combine the ether solution, evaporate to dryness, add 2ml of chloroform to the residue to dissolve, and use it as the test solution; take another 0.5g of sub-rhubarb reference drug, add 55ml of methanol, sonicate for 35min, filter, and take the filtrate 25ml, evaporate to dryness, add 30ml of water to the residue to dissolve, add 3ml of hydrochloric acid, heat and reflux for 35min, cool, extract with ether 3 times, 40ml each time, combine the ether solution, evaporate to dryness, add 4ml of chloroform to dissolve the residue, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com