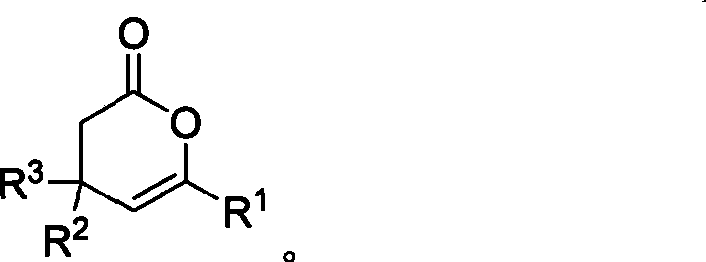
Method for synthesizing 4,6-substituted 3,4- dihydro-pyran-2-ketone derivative
A derivative and dihydro technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as harsh reaction conditions, difficult operation, and difficult control. Achieve mild reaction conditions, easy operation and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
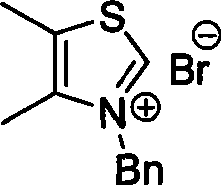
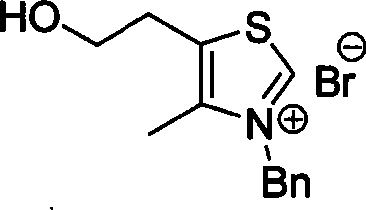
[0026] Embodiment 1: Preparation of thiazole nitrogen heterocyclic carbene precursor salt
[0027] Under the protection of argon at room temperature, in a dry reaction tube, dissolve 4,5-dimethylthiazole or 4-methyl-5-hydroxyethylthiazole (1.0mmol) in 10mL of dry acetonitrile. Under certain conditions, (1.0 mmol) benzyl bromide or benzyl chloride was slowly added dropwise to the system, and refluxed for 3 hours. After the reaction was completed, it was naturally cooled to room temperature, and under rapid stirring, 30 mL of ethyl acetate was slowly added dropwise to the system, and a large amount of white solids precipitated out of the system, which was then left to stand. After filtration, a white solid was obtained, which was the precursor salt of thiazole carbenes.
[0028] C1: bromide (3-benzyl-4,5-dimethylthiazole)
[0029] 3-Benzyl-4,5-dimethylthiazolium bromide
[0030]
[0031] Solid, 91% yield. 'H NMR (300MHZ, DMSO) 6 more or less 7.492-7.324 (m, 5H), 5.809 (s,...
Embodiment 2
[0061] Embodiment 2: optimization of catalyst and reaction conditions
[0062] Table 1. Screening of nitrogen heterocyclic carbene catalysts a
[0063]
[0064]
[0065] a 1 Condition: 7a (0.2mmol), 20mol% catalyst., and 30mol% 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), tetrahydrofuran (THF) (1.0mL), 40 ℃. b use 1 H NMR determination. c Yield (Isolated yield).
[0066] Table2. Screening of reaction conditions α
[0067]
[0068]
[0069]
[0070] α Reaction conditions: 7a (0.2 mmol), x mol% 5, and y mol% 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), in solvent (1.0 mL).
[0071] b Isolated yields. Wherein, DCM is dichloromethane, toluene is toluene, EtOAc is ethyl acetate, DMSO is dimethyl sulfoxide, and dioxane is dioxane.
Embodiment 3
[0072] Example 3: Synthesis of 3,4-dihydropyran-2-ones
[0073]
[0074] Under the protection of argon at room temperature, in a dry reaction tube, 2-acyl-1-formylcyclopropane 7 (1.0 mmol), thiazolium salt 5 (0.05 mmol), DBU (45 μL), Molecular sieves (100 mg), dissolved in 5 mL of dry 1,4-dioxane, stirred at 65 ° C, after the reaction was completed, naturally cooled to room temperature, filtered with diatomaceous earth, washed with dichloromethane, evaporated to dryness, column Chromatography (diethyl ether / petroleum ether=1 / 4-1 / 8) yielded compound 8.
[0075] P1: 4,6-diphenyl-3,4-dihydropyran-2-one
[0076] 4,6-diphenyl-3,4-dihydropyran-2-one
[0077]
[0078] Colorless oily liquid, 92% yield. 1 H NMR (CDCl 3 , 300MHz) δ 2.80 (dd, 1H, J 1 =8.7Hz,J 2 =15.9Hz), 3.04(dd,1H,J 1 =6.6Hz,J 2 =15.9Hz), 3.98(m, 1H), 5.97(d, 1H, J=3.9Hz), 7.26-7.44(m, 8H), 7.66-7.69(m, 2H); 13 C NMR (75MHz, CDCl 3 ): δ 36.9, 37.2, 104.2, 124.6, 127.0, 127.5, 128.5, 129.1, 129.2, 132.1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com