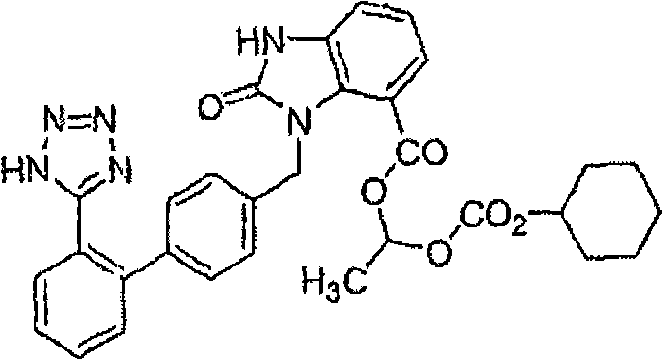
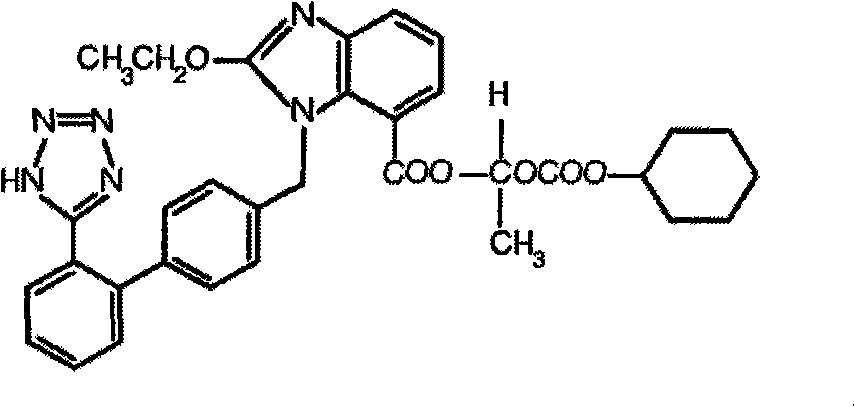
Stable candesartan cilexetil amlodipine pharmaceutical composition and its preparation method
A technology of candesartan cilexetil and amlodipine, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations of non-active ingredients, etc., can solve the problem of decreased stability of amlodipine, without consideration of candesartan and amlodipine Dipine interaction and stability issues, increased oxidative degradation impurities, etc., to achieve the effects of shortening tablet production time, reducing labor hours, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The following are specific examples of the present invention, but it does not mean that the present invention is limited to the following examples. Embodiment 1 (candesartan cilexetil 8mg / amlodipine 5mg)
[0038]
[0039] Preparation process (direct powder compression): Amlodipine besylate, candesartan cilexetil, mannitol, microcrystalline cellulose, hydroxypropylmethylcellulose, croscarmellose sodium, Polyethylene glycol 6000, yellow iron oxide, and magnesium stearate were mixed in a V-shaped blender for 15 minutes, and pressed into tablets with a rotary tablet machine, with a tablet weight of about 127mg.
Embodiment 2
[0040] Embodiment 2 (candesartan cilexetil 8mg / amlodipine 2.5mg)
[0041]
[0042] Preparation process (direct powder compression): the prescription amount of amlodipine besylate, candesartan cilexetil, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, sodium carboxymethyl starch, polyethylene glycol 4000. Mix yellow iron oxide and magnesium stearate in a multi-dimensional motion mixer for 10 minutes, and compress into tablets with a rotary tablet press, with a tablet weight of about 124mg.
Embodiment 3
[0043] Embodiment 3 (candesartan cilexetil 8mg / amlodipine 5mg)
[0044]
[0045]
[0046] Preparation process (direct powder compression): the prescribed amount of amlodipine besylate, candesartan cilexetil, lactose, pregelatinized starch, hydroxypropyl cellulose, crospovidone, polyethylene glycol 6000 , yellow iron oxide and magnesium stearate were mixed in a high-speed stirring granulator for 15 minutes, and then compressed by a rotary tablet machine, with a tablet weight of about 122mg. Embodiment 4 (candesartan cilexetil 8mg / amlodipine 5mg)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com